IL4I1-catalyzed tryptophan metabolites mediate the anti-inflammatory function of cytokine-primed human muscle stem cells
- PMID: 37507432
- PMCID: PMC10382538
- DOI: 10.1038/s41420-023-01568-x
IL4I1-catalyzed tryptophan metabolites mediate the anti-inflammatory function of cytokine-primed human muscle stem cells
Abstract
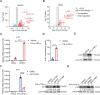
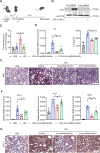
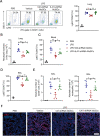
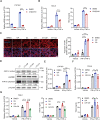
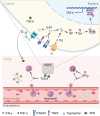
Muscle stem cells (MuSCs) have been demonstrated to exert impressive therapeutic efficacy in disease settings through orchestrating inflammatory microenvironments. Nevertheless, the mechanisms underlying the immunoregulatory property of MuSCs remain largely uncharacterized. Here, we showed that interleukin-4-induced-1 (IL4I1), an essential enzyme that catalyzes indole metabolism in humans, was highly expressed in human MuSCs exposed to IFN-γ and TNF-α. Functionally, the MuSCs were found to inhibit the infiltration of neutrophils into sites of inflammation in a IL4I1-dependent manner and thus ameliorate acute lung injury in mice. Mechanistically, the indole metabolites, including indole-3-pyruvic acid (I3P) and indole-3-aldehyde (I3A), produced by IL4I1, acted as ligands to activate aryl hydrocarbon receptor (AHR), leading to augmented expression of TNF-stimulated gene 6 (TSG-6) in inflammatory cytokine-primed MuSCs. Furthermore, I3P administration alone suppressed neutrophil infiltration into damaged lungs. I3P could also reduce the level of reactive oxygen species in neutrophils. Therefore, our study has uncovered a novel mechanism by which MuSCs acquire their immunoregulatory property and may help to develop or optimize MuSC-based therapies for inflammatory diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
- 32150710523/National Natural Science Foundation of China (National Science Foundation of China)
- 82202032/National Natural Science Foundation of China (National Science Foundation of China)
- 2021YFA1100600/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2022YFA0807300/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
LinkOut - more resources
Full Text Sources
Miscellaneous

