5-Aminosalicylic acid alters the gut microbiota and altered microbiota transmitted vertically to offspring have protective effects against colitis
- PMID: 37507482
- PMCID: PMC10382598
- DOI: 10.1038/s41598-023-39491-x
5-Aminosalicylic acid alters the gut microbiota and altered microbiota transmitted vertically to offspring have protective effects against colitis
Abstract
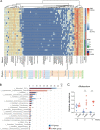
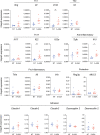
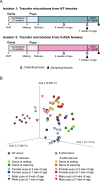
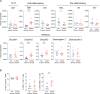
Although many therapeutic options are available for inflammatory bowel disease (IBD), 5-aminosalicylic acid (5-ASA) is still the key medication, particularly for ulcerative colitis (UC). However, the mechanism of action of 5-ASA remains unclear. The intestinal microbiota plays an important role in the pathophysiology of IBD, and we hypothesized that 5-ASA alters the intestinal microbiota, which promotes the anti-inflammatory effect of 5-ASA. Because intestinal inflammation affects the gut microbiota and 5-ASA can change the severity of inflammation, assessing the impact of inflammation and 5-ASA on the gut microbiota is not feasible in a clinical study of patients with UC. Therefore, we undertook a translational study to demonstrate a causal link between 5-ASA administration and alterations of the intestinal microbiota. Furthermore, by rigorously controlling environmental confounders and excluding the effect of 5-ASA itself with a vertical transmission model, we observed that the gut microbiota altered by 5-ASA affected host mucosal immunity and decreased susceptibility to dextran sulfate sodium-induce colitis. Although the potential intergenerational transmission of epigenetic changes needs to be considered in this study, these findings suggested that alterations in the intestinal microbiota induced by 5-ASA directed the host immune system towards an anti-inflammatory state, which underlies the mechanism of 5-ASA efficacy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

