Alterations in immune cell phenotype and cytotoxic capacity in HER2+ breast cancer patients receiving HER2-targeted neo-adjuvant therapy
- PMID: 37507543
- PMCID: PMC10491671
- DOI: 10.1038/s41416-023-02375-y
Alterations in immune cell phenotype and cytotoxic capacity in HER2+ breast cancer patients receiving HER2-targeted neo-adjuvant therapy
Abstract
Background: The phase II neo-adjuvant clinical trial ICORG10-05 (NCT01485926) compared chemotherapy in combination with trastuzumab, lapatinib or both in patients with HER2+ breast cancer. We studied circulating immune cells looking for alterations in phenotype, genotype and cytotoxic capacity (direct and antibody-dependent cell-mediated cytotoxicity (ADCC)) in the context of treatment response.
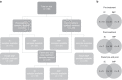
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from pre- (n = 41) and post- (n = 25) neo-adjuvant treatment blood samples. Direct/trastuzumab-ADCC cytotoxicity of patient-derived PBMCs against K562/SKBR3 cell lines was determined ex vivo. Pembrolizumab was interrogated in 21 pre-treatment PBMC ADCC assays. Thirty-nine pre-treatment and 21 post-treatment PBMC samples were immunophenotyped. Fc receptor genotype, tumour infiltrating lymphocyte (TIL) levels and oestrogen receptor (ER) status were quantified.
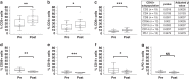
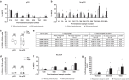
Results: Treatment attenuated the cytotoxicity/ADCC of PBMCs. CD3+/CD4+/CD8+ T cells increased following therapy, while CD56+ NK cells/CD14+ monocytes/CD19+ B cells decreased with significant post-treatment immune cell changes confined to patients with residual disease. Pembrolizumab-augmented ex vivo PBMC ADCC activity was associated with residual disease, but not pathological complete response. Pembrolizumab-responsive PBMCs were associated with lower baseline TIL levels and ER+ tumours.
Conclusions: PBMCs display altered phenotype and function following completion of neo-adjuvant treatment. Anti-PD-1-responsive PBMCs in ex vivo ADCC assays may be a biomarker of treatment response.
© 2023. The Author(s).
Conflict of interest statement
JC has received honoraria from Pfizer, MSD Oncology, Pierre Fabre, and AstraZeneca; has acted in a Consulting or Advisory Role for AstraZeneca, Novartis, MSD, Cepheid and received Speakers' Bureau from Pfizer; has received research funding from Boehringer Ingelheim, Roche, Puma Biotechnology Regeneron, Novartis, MSD Oncology, BMS GmbH, and Co. KG; has received travel, accommodations, and expenses/conference registration from Roche, Daiichi Sankyo, AstraZeneca, MSD, Pfizer, Novartis, and Regeneron; is the Chief Medical Officer (CMO) at OncoMark Ltd and is employed with OncoAssure Ltd, and has associated stock and ownership interests with these entities. MSJM is a co-founder of and shareholder in TORL Biotherapeutics LLC and 1200 Pharma LLC. DMC has received research funding and research materials from Roche/Genentech, WntResearch; research materials from Sanofi; and has received research funding and consultancy fees from Puma Biotechnology, Inc. DMC, NG, NO, and JC report the filing of patent application WO2020011770A1—A method of predicting response to treatment in cancer patients. All other authors have declared no conflicts of interest.
Figures



References
-
- Gómez Román VR, Murray JC, Weiner LM. Antibody-dependent cellular cytotoxicity (ADCC). Antibody Fc. 2014:1–27.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

