The role of pyruvate-induced enhancement of oxygen metabolism in extracellular purinergic signaling in the post-cardiac arrest rat model
- PMID: 37507639
- PMCID: PMC11303634
- DOI: 10.1007/s11302-023-09958-7
The role of pyruvate-induced enhancement of oxygen metabolism in extracellular purinergic signaling in the post-cardiac arrest rat model
Abstract
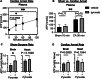
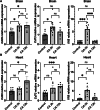
Purine nucleotide adenosine triphosphate (ATP) is a source of intracellular energy maintained by mitochondrial oxidative phosphorylation. However, when released from ischemic cells into the extracellular space, they act as death-signaling molecules (eATP). Despite there being potential benefit in using pyruvate to enhance mitochondria by inducing a highly oxidative metabolic state, its association with eATP levels is still poorly understood. Therefore, while we hypothesized that pyruvate could beneficially increase intracellular ATP with the enhancement of mitochondrial function after cardiac arrest (CA), our main focus was whether a proportion of the raised intracellular ATP would detrimentally leak out into the extracellular space. As indicated by the increased levels in systemic oxygen consumption, intravenous administrations of bolus (500 mg/kg) and continuous infusion (1000 mg/kg/h) of pyruvate successfully increased oxygen metabolism in post 10-min CA rats. Plasma ATP levels increased significantly from 67 ± 11 nM before CA to 227 ± 103 nM 2 h after the resuscitation; however, pyruvate administration did not affect post-CA ATP levels. Notably, pyruvate improved post-CA cardiac contraction and acidemia (low pH). We also found that pyruvate increased systemic CO2 production post-CA. These data support that pyruvate has therapeutic potential for improving CA outcomes by enhancing oxygen and energy metabolism in the brain and heart and attenuating intracellular hydrogen ion disorders, but does not exacerbate the death-signaling of eATP in the blood.
Keywords: Adenosine triphosphate; Energy metabolism; Gas exchange; Mitochondria; Oxygen consumption; Pyruvate.
© 2023. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Shinozaki and Becker own intellectual property of metabolic measurement in critically ill patients. Shinozaki has grant/research supported by Nihon Kohden Corp. Becker has grant/research supported by Philips Healthcare, the National Institutes of Health, Nihon Kohden Corp., BeneChill Inc., Zoll Medical Corp, Medtronic Foundation, and patents in the areas of hypothermia induction and perfusion therapies. The other authors have no disclosures.
Figures





References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM et al (2011) Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 123:e18–e209 - PMC - PubMed
-
- Neumar RW, Eigel B, Callaway CW, Estes NA 3rd, Jollis JG, Kleinman ME, Morrison L, Peberdy MA, Rabinstein A, Rea TD, Sendelbach S (2015) The American Heart Association response to the 2015 Institute of Medicine Report on strategies to improve cardiac arrest survival. Circulation 132:1049–1070 10.1161/CIR.0000000000000233 - DOI - PubMed
-
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP et al (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79:350–379 10.1016/j.resuscitation.2008.09.017 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

