Menstrual blood-derived mesenchymal stromal cells: impact of preconditioning on the cargo of extracellular vesicles as potential therapeutics
- PMID: 37507751
- PMCID: PMC10386225
- DOI: 10.1186/s13287-023-03413-5
Menstrual blood-derived mesenchymal stromal cells: impact of preconditioning on the cargo of extracellular vesicles as potential therapeutics
Abstract
Background: Mesenchymal stromal cells (MSCs) have been shown to exert their therapeutic effects through the secretion of broad spectrum of paracrine factors, including extracellular vesicles (EVs). Accordingly, EVs are being pursued as a promising alternative to cell-based therapies. Menstrual blood-derived stromal cells (MenSCs) are a type of MSC that, due to their immunomodulatory and regenerative properties, have emerged as an innovative source. Additionally, new strategies of cell priming may potentially alter the concentration and cargo of released EVs, leading to modification of their biological properties. In this study, we aimed to characterize the EVs released by MenSCs and compare their therapeutic potential under three different preconditioning conditions (proinflammatory stimuli, physioxia, and acute hypoxia).
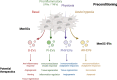
Methods: MenSCs were isolated from five healthy women. Following culturing to 80% confluence, MenSCs were exposed to different priming conditions: basal (21% O2), proinflammatory stimuli (IFNγ and TNFα, 21% O2), physioxia (1-2% O2), and acute hypoxia (< 1% O2) for 48-72 h. Conditioned media from MenSCs was collected after 48 h and EVs were isolated by a combination of ultra-filtration and differential centrifugation. An extensive characterization ranging from nano-flow cytometry (nFC) to quantitative high-throughput shotgun proteomics was performed. Bioinformatics analyses were used to derive hypotheses on their biological properties.
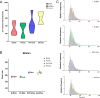
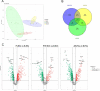
Results: No differences in the morphology, size, or number of EVs released were detected between priming conditions. The proteome analysis associated with basal MenSC-EVs prominently revealed their immunomodulatory and regenerative capabilities. Furthermore, quantitative proteomic analysis of differentially produced MenSC-EVs provided sufficient evidence for the utility of the differential preconditioning in purpose-tailoring EVs for their therapeutic application: proinflammatory priming enhanced the anti-inflammatory, regenerative and immunomodulatory capacity in the innate response of EVs, physioxia priming also improves tissue regeneration, angiogenesis and their immunomodulatory capacity targeting on the adaptive response, while acute hypoxia priming, increased hemostasis and apoptotic processes regulation in MenSC-EVs, also by stimulating immunomodulation mainly through the adaptive response.
Conclusions: Priming of MenSCs under proinflammatory and hypoxic conditions affected the cargo proteome of EVs released, resulting in different therapeutic potential, and thus warrants experimental exploration with the aim to generate better-defined MSC-derived bioproducts.
Keywords: Exosomes; Extracellular vesicles (EVs); High-throughput proteomics; Menstrual blood; Mesenchymal stromal cells (MSCs); Microvesicles; Preconditioning.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

