Psychoactive drugs and male fertility: impacts and mechanisms
- PMID: 37507788
- PMCID: PMC10375764
- DOI: 10.1186/s12958-023-01098-2
Psychoactive drugs and male fertility: impacts and mechanisms
Abstract
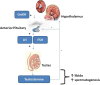
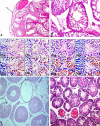
Although psychoactive drugs have their therapeutic values, they have been implicated in the pathogenesis of male infertility. This study highlights psychoactive drugs reported to impair male fertility, their impacts, and associated mechanisms. Published data from scholarly peer-reviewed journals were used for the present study. Papers were assessed through AJOL, DOAJ, Google Scholar, PubMed/PubMed Central, and Scopus using Medical Subjects Heading (MeSH) indexes and relevant keywords. Psychoactive drugs negatively affect male reproductive functions, including sexual urge, androgen synthesis, spermatogenesis, and sperm quality. These drugs directly induce testicular toxicity by promoting ROS-dependent testicular and sperm oxidative damage, inflammation, and apoptosis, and they also suppress the hypothalamic-pituitary-testicular axis. This results in the suppression of circulating androgen, impaired spermatogenesis, and reduced sperm quality. In conclusion, psychoactive drug abuse not only harms male sexual and erectile function as well as testicular functions, viz., testosterone concentration, spermatogenesis, and sperm quality, but it also alters testicular histoarchitecture through a cascade of events via multiple pathways. Therefore, offering adequate and effective measures against psychoactive drug-induced male infertility remains pertinent.
Keywords: Cannabis; Depressants; Drug abuse; Hallucinogens; Male infertility; Marijuana; Narcotics; Opioids; Psychoactive drugs; Stimulants.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

