How clinicians discuss patients' donor registrations of consent and presumed consent in donor conversations in an opt-out system: a qualitative embedded multiple-case study
- PMID: 37507800
- PMCID: PMC10375668
- DOI: 10.1186/s13054-023-04581-9
How clinicians discuss patients' donor registrations of consent and presumed consent in donor conversations in an opt-out system: a qualitative embedded multiple-case study
Abstract
Background: The Netherlands introduced an opt-out donor system in 2020. While the default in (presumed) consent cases is donation, family involvement adds a crucial layer of influence when applying this default in clinical practice. We explored how clinicians discuss patients' donor registrations of (presumed) consent in donor conversations in the first years of the opt-out system.
Methods: A qualitative embedded multiple-case study in eight Dutch hospitals. We performed a thematic analysis based on audio recordings and direct observations of donor conversations (n = 15, 7 consent and 8 presumed consent) and interviews with the clinicians involved (n = 16).
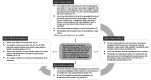
Results: Clinicians' personal considerations, their prior experiences with the family and contextual factors in the clinicians' profession defined their points of departure for the conversations. Four routes to discuss patients' donor registrations were constructed. In the Consent route (A), clinicians followed patients' explicit donation wishes. With presumed consent, increased uncertainty in interpreting the donation wish appeared and prompted clinicians to refer to "the law" as a conversation starter and verify patients' wishes multiple times with the family. In the Presumed consent route (B), clinicians followed the law intending to effectuate donation, which was more easily achieved when families recognised and agreed with the registration. In the Consensus route (C), clinicians provided families some participation in decision-making, while in the Family consent route (D), families were given full decisional capacity to pursue optimal grief processing.
Conclusion: Donor conversations in an opt-out system are a complex interplay between seemingly straightforward donor registrations and clinician-family interactions. When clinicians are left with concerns regarding patients' consent or families' coping, families are given a larger role in the decision. A strict uniform application of the opt-out system is unfeasible. We suggest incorporating the four previously described routes in clinical training, stimulating discussions across cases, and encouraging public conversations about donation.
Keywords: Communication; End-of-life decision-making; Intensive care; Medical ethics; Opt-out consent; Organ donation; Professional-family relations; Qualitative research.
© 2023. The Author(s).
Conflict of interest statement
All authors have completed a disclosure form based on uniform ICMJE guidelines. As potential participants, donation intensivists did not have final decisional rights regarding the results and discussion sections of the manuscript. The authors have no competing interests to declare.
Figures

References
-
- Wet op de orgaandonatie: Volksgezondheid, Welzijn en Sport (2022). Available from: https://wetten.overheid.nl/BWBR0008066/2022-01-01/ (accessed 28-09-2022).
MeSH terms
LinkOut - more resources
Full Text Sources

