Regulation of Redox Profile and Genomic Instability by Physical Exercise Contributes to Neuroprotection in Mice with Experimental Glioblastoma
- PMID: 37507883
- PMCID: PMC10376052
- DOI: 10.3390/antiox12071343
Regulation of Redox Profile and Genomic Instability by Physical Exercise Contributes to Neuroprotection in Mice with Experimental Glioblastoma
Abstract
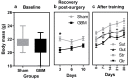
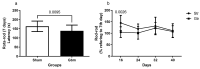
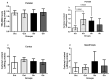
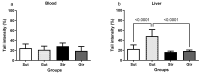
Glioblastoma (GBM) is an aggressive, common brain cancer known to disrupt redox biology, affecting behavior and DNA integrity. Past research remains inconclusive. To further understand this, an investigation was conducted on physical training's effects on behavior, redox balance, and genomic stability in GBMA models. Forty-seven male C57BL/6J mice, 60 days old, were divided into GBM and sham groups (n = 15, n = 10, respectively), which were further subdivided into trained (Str, Gtr; n = 10, n = 12) and untrained (Sut, Gut; n = 10, n = 15) subsets. The trained mice performed moderate aerobic exercises on a treadmill five to six times a week for a month while untrained mice remained in their enclosures. Behavior was evaluated using open-field and rotarod tests. Post training, the mice were euthanized and brain, liver, bone marrow, and blood samples were analyzed for redox and genomic instability markers. The results indicated increased latency values in the trained GBM (Gtr) group, suggesting a beneficial impact of exercise. Elevated reactive oxygen species in the parietal tissue of untrained GBM mice (Gut) were reduced post training. Moreover, Gtr mice exhibited lower tail intensity, indicating less genomic instability. Thus, exercise could serve as a promising supplemental GBM treatment, modulating redox parameters and reducing genomic instability.
Keywords: brain tumor; genomic stability; glioblastoma; oxidative stress; physical exercise.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

