Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota
- PMID: 37507906
- PMCID: PMC10376639
- DOI: 10.3390/antiox12071366
Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota
Abstract
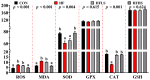
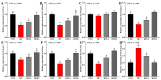
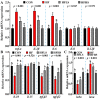
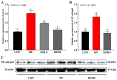
An eight-week trial was conducted to investigate the effects of sanguinarine supplementation (600 μg and 1200 μg/kg) in high-fat (crude fat: 10%) diets (HF) on the intestinal physiological function of Ctenopharyngodon idellus (initial weight 50.21 ± 0.68 g), based on a basic diet (5% crude fat, CON), which were named HFLS and HFHS, respectively. The results showed that the HF diet significantly impaired the intestinal immune and physical barrier function, and disrupted the balance of the intestinal microbiota in grass carp. Compared to the HF diet, sanguinarine supplementation significantly improved the levels of serum C4, C3, AKP, IgA, and IgM, and enhanced the intestinal antioxidant capacity (gr, CuZnsod, gpx4, cat, gsto, and nrf2 expression were significantly up-regulated). Sanguinarine significantly down-regulated the expression of claudin-15 and up-regulated the expression of claudin-b, claudin-c, occludin, and zo-1 by inhibiting MLCK signaling molecules. Additionally, sanguinarine significantly down-regulated the expression of il-6, il-1β, and tnf-α and up-regulated the expression of il-10, tgf-β2, and tgf-β1 by inhibiting NF-κB signaling molecules, thereby alleviating intestinal inflammation caused by HF diets. Furthermore, compared to the HF diet, the abundance of Fusobacterium and Cetobacterium in the HFHS diet increased significantly, while the abundance of Firmicutes and Streptococcus showed the opposite trend. In conclusion, the HF diet had a negative impact on grass carp, while sanguinarine supplementation enhanced intestinal antioxidant ability, alleviated intestinal barrier damage, and ameliorated the homeostasis of the intestinal microbiota.
Keywords: Chinese herbal extract; Ctenopharyngodon idellus; high-fat diet; intestinal barrier; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Impact of exogenous lipase supplementation on growth, intestinal function, mucosal immune and physical barrier, and related signaling molecules mRNA expression of young grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2016 Aug;55:88-105. doi: 10.1016/j.fsi.2016.05.006. Epub 2016 May 7. Fish Shellfish Immunol. 2016. PMID: 27164217
-
Deficiency of dietary niacin impaired intestinal mucosal immune function via regulating intestinal NF-κB, Nrf2 and MLCK signaling pathways in young grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2016 Feb;49:177-93. doi: 10.1016/j.fsi.2015.12.015. Epub 2015 Dec 13. Fish Shellfish Immunol. 2016. PMID: 26693667
-
Sodium butyrate enhanced physical barrier function referring to Nrf2, JNK and MLCK signaling pathways in the intestine of young grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2018 Feb;73:121-132. doi: 10.1016/j.fsi.2017.12.009. Epub 2017 Dec 5. Fish Shellfish Immunol. 2018. PMID: 29222028
-
Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-κB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare.Fish Shellfish Immunol. 2016 Nov;58:177-192. doi: 10.1016/j.fsi.2016.09.029. Epub 2016 Sep 15. Fish Shellfish Immunol. 2016. PMID: 27640333
-
Exogenous phospholipids supplementation improves growth and modulates immune response and physical barrier referring to NF-κB, TOR, MLCK and Nrf2 signaling factors in the intestine of juvenile grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2015 Nov;47(1):46-62. doi: 10.1016/j.fsi.2015.08.024. Epub 2015 Aug 22. Fish Shellfish Immunol. 2015. PMID: 26306855
Cited by
-
Nutritional and physiological effects of high-fat diets in finfish: effects on growth, immunity, lipid metabolism, and intestinal health: a review.J Comp Physiol B. 2025 Aug;195(4):415-437. doi: 10.1007/s00360-025-01626-z. Epub 2025 Jul 21. J Comp Physiol B. 2025. PMID: 40689965 Review.
-
L-Carnitine Improves Muscle Nutrient Metabolism and Intestinal Health in High-Fat-Fed Carp (Cyprinus carpio).Aquac Nutr. 2025 Jan 9;2025:5623889. doi: 10.1155/anu/5623889. eCollection 2025. Aquac Nutr. 2025. PMID: 39822797 Free PMC article.
-
Yellow mealworm (Tenebrio molitor) meal in diets of grass carp (Ctenopharyngodon idellus): Effects on growth performance, antioxidant capacity, immunity, intestinal morphology, and intestinal microbiota.Anim Nutr. 2025 Jan 29;21:70-83. doi: 10.1016/j.aninu.2025.01.001. eCollection 2025 Jun. Anim Nutr. 2025. PMID: 40416717 Free PMC article.
-
Sanguinarine-Chelerythrine from Coptis chinensis Offers Analgesic and Anti-Inflammatory Effects Without Gastrotoxicity.Pharmaceutics. 2025 Mar 2;17(3):323. doi: 10.3390/pharmaceutics17030323. Pharmaceutics. 2025. PMID: 40142987 Free PMC article.
-
High-fat diet may increase the risk of insulin resistance by inducing dysbiosis.Metabol Open. 2025 Jul 22;27:100381. doi: 10.1016/j.metop.2025.100381. eCollection 2025 Sep. Metabol Open. 2025. PMID: 40741424 Free PMC article. Review.
References
-
- Obling J.M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012;20:601–602. doi: 10.1007/s10499-011-9480-6. - DOI
-
- Li X.F., Liu W.B., Jiang Y.Y., Zhu H., Ge X.P. Effects of dietary protein and lipid levels in practical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture. 2010;303:65–70. doi: 10.1016/j.aquaculture.2010.03.014. - DOI
-
- Gao W., Liu Y.J., Tian L.X., Mai K.S., Liang G.Y., Yang H.J., Huai M.Y., Luo W.J. Protein-sparing capability of dietary lipid in herbivorous and omnivorous freshwater in fish: A comparative case study on grass carp (Ctenopharyngodon idella) and tilapia (Oreochromis niloticus × O. aureus) Aquacult. Nutr. 2011;17:2–12. doi: 10.1111/j.1365-2095.2009.00698.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

