Antioxidative Role of Heterophagy, Autophagy, and Mitophagy in the Retina and Their Association with the Age-Related Macular Degeneration (AMD) Etiopathogenesis
- PMID: 37507908
- PMCID: PMC10376332
- DOI: 10.3390/antiox12071368
Antioxidative Role of Heterophagy, Autophagy, and Mitophagy in the Retina and Their Association with the Age-Related Macular Degeneration (AMD) Etiopathogenesis
Abstract
Age-related macular degeneration (AMD), an oxidative stress-linked neurodegenerative disease, leads to irreversible damage of the central retina and severe visual impairment. Advanced age and the long-standing influence of oxidative stress and oxidative cellular damage play crucial roles in AMD etiopathogenesis. Many authors emphasize the role of heterophagy, autophagy, and mitophagy in maintaining homeostasis in the retina. Relevantly modifying the activity of both macroautophagy and mitophagy pathways represents one of the new therapeutic strategies in AMD. Our review provides an overview of the antioxidative roles of heterophagy, autophagy, and mitophagy and presents associations between dysregulations of these molecular mechanisms and AMD etiopathogenesis. The authors performed an extensive analysis of the literature, employing PubMed and Google Scholar, complying with the 2013-2023 period, and using the following keywords: age-related macular degeneration, RPE cells, reactive oxygen species, oxidative stress, heterophagy, autophagy, and mitophagy. Heterophagy, autophagy, and mitophagy play antioxidative roles in the retina; however, they become sluggish and dysregulated with age and contribute to AMD development and progression. In the retina, antioxidative roles also play in RPE cells, NFE2L2 and PGC-1α proteins, NFE2L2/PGC-1α/ARE signaling cascade, Nrf2 factor, p62/SQSTM1/Keap1-Nrf2/ARE pathway, circulating miRNAs, and Yttrium oxide nanoparticles performed experimentally in animal studies.
Keywords: RPE cells; age-related macular degeneration; autophagy; heterophagy; mitophagy; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

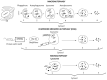

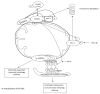
Similar articles
-
Mitochondrial quality control in AMD: does mitophagy play a pivotal role?Cell Mol Life Sci. 2018 Aug;75(16):2991-3008. doi: 10.1007/s00018-018-2843-7. Epub 2018 May 18. Cell Mol Life Sci. 2018. PMID: 29777261 Free PMC article. Review.
-
Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration.Autophagy. 2013 Jul;9(7):973-84. doi: 10.4161/auto.24546. Epub 2013 Apr 9. Autophagy. 2013. PMID: 23590900 Free PMC article. Review.
-
Potential of Telomerase in Age-Related Macular Degeneration-Involvement of Senescence, DNA Damage Response and Autophagy and a Key Role of PGC-1α.Int J Mol Sci. 2021 Jul 3;22(13):7194. doi: 10.3390/ijms22137194. Int J Mol Sci. 2021. PMID: 34281248 Free PMC article. Review.
-
Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress.Oxid Med Cell Longev. 2020 Aug 8;2020:2896036. doi: 10.1155/2020/2896036. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32831993 Free PMC article. Review.
-
Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration.Redox Biol. 2019 Jan;20:1-12. doi: 10.1016/j.redox.2018.09.011. Epub 2018 Sep 14. Redox Biol. 2019. PMID: 30253279 Free PMC article.
Cited by
-
Lamp2 Deficiency Enhances Susceptibility to Oxidative Stress-Induced RPE Degeneration.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):2. doi: 10.1167/iovs.66.2.2. Invest Ophthalmol Vis Sci. 2025. PMID: 39898910 Free PMC article.
-
Potential application of traditional Chinese medicine in age-related macular degeneration-focusing on mitophagy.Front Pharmacol. 2024 May 17;15:1410998. doi: 10.3389/fphar.2024.1410998. eCollection 2024. Front Pharmacol. 2024. PMID: 38828456 Free PMC article. Review.
-
Liraglutide Attenuates FFA-Induced Retinal Pigment Epithelium Dysfunction via AMPK Activation and Lipid Homeostasis Regulation in ARPE-19 Cells.Int J Mol Sci. 2025 Apr 14;26(8):3704. doi: 10.3390/ijms26083704. Int J Mol Sci. 2025. PMID: 40332323 Free PMC article.
-
Potential of autophagy in subretinal fibrosis in neovascular age-related macular degeneration.Cell Mol Biol Lett. 2025 Apr 30;30(1):54. doi: 10.1186/s11658-025-00732-8. Cell Mol Biol Lett. 2025. PMID: 40307700 Free PMC article. Review.
-
Sialic Acid Mimetic Microglial Sialic Acid-Binding Immunoglobulin-like Lectin Agonism: Potential to Restore Retinal Homeostasis and Regain Visual Function in Age-Related Macular Degeneration.Pharmaceuticals (Basel). 2023 Dec 16;16(12):1735. doi: 10.3390/ph16121735. Pharmaceuticals (Basel). 2023. PMID: 38139861 Free PMC article. Review.
References
-
- Salimiaghdam N., Riazi-Esfahani M., Fukuhara P.S., Schneider K., Kenney M.C. Age-related Macular Degeneration (AMD): A Review on its Epidemiology and Risk Factors. Open Ophthalmol. J. 2019;13:90–99. doi: 10.2174/1874364101913010090. - DOI
-
- Chong E.W., Wang Y., Robman L.D., Aung K.Z., Makeyeva G.A., Giles G.G., Graves S., Cicuttini F.M., Guymer R.H. Age related macular degeneration and total hip replacement due to osteoarthritis or fracture: Melbourne Collaborative Cohort Study. PLoS ONE. 2015;10:e0137322. doi: 10.1371/journal.pone.0137322. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

