Hyperforin Enhances Heme Oxygenase-1 Expression Triggering Lipid Peroxidation in BRAF-Mutated Melanoma Cells and Hampers the Expression of Pro-Metastatic Markers
- PMID: 37507910
- PMCID: PMC10376533
- DOI: 10.3390/antiox12071369
Hyperforin Enhances Heme Oxygenase-1 Expression Triggering Lipid Peroxidation in BRAF-Mutated Melanoma Cells and Hampers the Expression of Pro-Metastatic Markers
Abstract
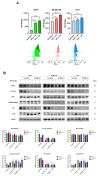
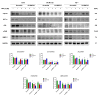
Hyperforin (HPF) is an acylphloroglucinol compound found abundantly in Hypericum perforatum extract which exhibits antidepressant, anti-inflammatory, antimicrobial, and antitumor activities. Our recent study revealed a potent antimelanoma effect of HPF, which hinders melanoma cell proliferation, motility, colony formation, and induces apoptosis. Furthermore, we have identified glutathione peroxidase-4 (GPX-4), a key enzyme involved in cellular protection against iron-induced lipid peroxidation, as one of the molecular targets of HPF. Thus, in three BRAF-mutated melanoma cell lines, we investigated whether iron unbalance and lipid peroxidation may be a part of the molecular mechanisms underlying the antimelanoma activity of HPF. Initially, we focused on heme oxygenase-1 (HO-1), which catalyzes the heme group into CO, biliverdin, and free iron, and observed that HPF treatment triggered the expression of this inducible enzyme. In order to investigate the mechanism involved in HO-1 induction, we verified that HPF downregulates the BTB and CNC homology 1 (BACH-1) transcription factor, an inhibitor of the heme oxygenase 1 (HMOX-1) gene transcription. Remarkably, we observed a partial recovery of cell viability and an increase in the expression of the phosphorylated and active form of retinoblastoma protein when we suppressed the HMOX-1 gene using HMOX-1 siRNA while HPF was present. This suggests that the HO-1 pathway is involved in the cytostatic effect of HPF in melanoma cells. To explore whether lipid peroxidation is induced, we conducted cytofluorimetric analysis and observed a significant increase in the fluorescence of the BODIPY C-11 probe 48 h after HPF administration in all tested melanoma cell lines. To discover the mechanism by which HPF triggers lipid peroxidation, along with the induction of HO-1, we examined the expression of additional proteins associated with iron homeostasis and lipid peroxidation. After HPF administration, we confirmed the downregulation of GPX-4 and observed low expression levels of SLC7A11, a cystine transporter crucial for the glutathione production, and ferritin, able to sequester free iron. A decreased expression level of these proteins can sensitize cells to lipid peroxidation. On the other hand, HPF treatment resulted in increased expression levels of transferrin, which facilitates iron uptake, and LC3B proteins, a molecular marker of autophagy induction. Indeed, ferritin and GPX-4 have been reported to be digested during autophagy. Altogether, these findings suggest that HPF induced lipid peroxidation likely through iron overloading and decreasing the expression of proteins that protect cells from lipid peroxidation. Finally, we examined the expression levels of proteins associated with melanoma cell invasion and metastatic potential. We observed the decreased expression of CD133, octamer-4, tyrosine-kinase receptor AXL, urokinase plasminogen activator receptor, and metalloproteinase-2 following HPF treatment. These findings provide further support for our previous observations, demonstrating the inhibitory effects of HPF on cell motility and colony formation in soft agar, which are both metastasis-related processes in tumor cells.
Keywords: BACH-1; CD133; FSP1; LC3B; MMP-2; NRF-2; ferritin; ferroptosis; heme oxigenase-1; uPAR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hyperforin Elicits Cytostatic/Cytotoxic Activity in Human Melanoma Cell Lines, Inhibiting Pro-Survival NF-κB, STAT3, AP1 Transcription Factors and the Expression of Functional Proteins Involved in Mitochondrial and Cytosolic Metabolism.Int J Mol Sci. 2023 Jan 9;24(2):1263. doi: 10.3390/ijms24021263. Int J Mol Sci. 2023. PMID: 36674794 Free PMC article.
-
Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death.Oncotarget. 2015 Sep 15;6(27):24393-403. doi: 10.18632/oncotarget.5162. Oncotarget. 2015. PMID: 26405158 Free PMC article.
-
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders.Antioxidants (Basel). 2022 Mar 15;11(3):555. doi: 10.3390/antiox11030555. Antioxidants (Basel). 2022. PMID: 35326205 Free PMC article. Review.
-
Spin trap (N-t-butyl-alpha-phenylnitrone)-mediated suprainduction of heme oxygenase-1 in kidney ischemia/reperfusion model: role of the oxygenase in protection against oxidative injury.J Pharmacol Exp Ther. 1999 Nov;291(2):911-9. J Pharmacol Exp Ther. 1999. PMID: 10525116
-
Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis.Int J Mol Sci. 2022 Dec 27;24(1):449. doi: 10.3390/ijms24010449. Int J Mol Sci. 2022. PMID: 36613888 Free PMC article. Review.
Cited by
-
Ferroptosis: a novel mechanism of cell death in ophthalmic conditions.Front Immunol. 2024 Jun 27;15:1440309. doi: 10.3389/fimmu.2024.1440309. eCollection 2024. Front Immunol. 2024. PMID: 38994366 Free PMC article. Review.
-
Hyperforin, the major metabolite of St. John's wort, exhibits pan-coronavirus antiviral activity.Front Microbiol. 2024 Aug 8;15:1443183. doi: 10.3389/fmicb.2024.1443183. eCollection 2024. Front Microbiol. 2024. PMID: 39176276 Free PMC article.
-
Lamivudine, Doravirine, and Cabotegravir Downregulate the Expression of Human Endogenous Retroviruses (HERVs), Inhibit Cell Growth, and Reduce Invasive Capability in Melanoma Cell Lines.Int J Mol Sci. 2024 Jan 28;25(3):1615. doi: 10.3390/ijms25031615. Int J Mol Sci. 2024. PMID: 38338893 Free PMC article.
-
The Multifaceted Roles of BACH1 in Disease: Implications for Biological Functions and Therapeutic Applications.Adv Sci (Weinh). 2025 Mar;12(10):e2412850. doi: 10.1002/advs.202412850. Epub 2025 Jan 30. Adv Sci (Weinh). 2025. PMID: 39887888 Free PMC article. Review.
-
Disruption of BACH1 Protects AC16 Cardiomyocytes Against Hypoxia/Reoxygenation-Evoked Injury by Diminishing CDKN3 Transcription.Cardiovasc Toxicol. 2024 Oct;24(10):1105-1115. doi: 10.1007/s12012-024-09900-2. Epub 2024 Jul 26. Cardiovasc Toxicol. 2024. PMID: 39060883
References
-
- Giorgi G., Mascaró M., Gandini N.A., Rabassa M.E., Coló G.P., Arévalo J., Curino A.C., Facchinetti M.M., Roque M.E. Iron Cycle Disruption by Heme Oxygenase-1 Activation Leads to a Reduced Breast Cancer Cell Survival. Biochim. Biophys. Acta—Mol. Basis Dis. 2023;1869:166621. doi: 10.1016/j.bbadis.2022.166621. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

