Antioxidant and Wound Healing Bioactive Potential of Extracts Obtained from Bark and Needles of Softwood Species
- PMID: 37507922
- PMCID: PMC10376860
- DOI: 10.3390/antiox12071383
Antioxidant and Wound Healing Bioactive Potential of Extracts Obtained from Bark and Needles of Softwood Species
Abstract
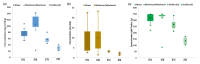
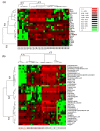
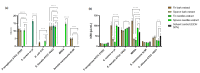
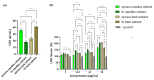
Interest in the extraction of phytochemical bioactive compounds, especially polyphenols from biomass, has recently increased due to their valuable biological potential as natural sources of antioxidants, which could be used in a wide range of applications, from foods and pharmaceuticals to green polymers and bio-based materials. The present research study aimed to provide a comprehensive chemical characterization of the phytochemical composition of forest biomass (bark and needles) of softwood species (Picea abies L., H. Karst., and Abies alba Mill.) and to investigate their in vitro antioxidant and antimicrobial activities to assess their potential in treating and healing infected chronic wounds. The DPPH radical-scavenging method and P-LD were used for a mechanistic explanation of the biomolecular effects of the investigated bioactive compounds. (+)-Catechin, epicatechin, rutin, myricetin, 4 hydroxybenzoic and p-cumaric acids, kaempherol, and apigenin were the main quantified polyphenols in coniferous biomass (in quantities around 100 µg/g). Also, numerous phenolic acids, flavonoids, stilbenes, terpenes, lignans, secoiridoids, and indanes with antioxidant, antimicrobial, anti-inflammatory, antihemolytic, and anti-carcinogenic potential were identified. The Abies alba needle extract was more toxic to microbial strains than the eukaryotic cells that provide its active wound healing principles. In this context, developing industrial upscaling strategies is imperative for the long-term success of biorefineries and incorporating them as part of a circular bio-economy.
Keywords: PI3Kγ; anti-hemolytic activity; antimicrobial activity; antioxidant activity; coniferous biomass; phytochemical bioactive compounds; polyphenols; protein-ligand docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Miranda I., Gominho J., Mirra I., Pereira H. Chemical Characterization of Barks from Picea Abies and Pinus Sylvestris after Fractioning into Different Particle Sizes. Ind. Crops Prod. 2012;36:395–400. doi: 10.1016/j.indcrop.2011.10.035. - DOI
-
- Mármol I., Quero J., Jiménez-Moreno N., Rodríguez-Yoldi M.J., Ancín-Azpilicueta C. A Systematic Review of the Potential Uses of Pine Bark in Food Industry and Health Care. Trends Food Sci. Technol. 2019;88:558–566. doi: 10.1016/j.tifs.2018.07.007. - DOI
-
- Kemppainen K., Siika-aho M., Pattathil S., Giovando S., Kruus K. Spruce Bark as an Industrial Source of Condensed Tannins and Non-Cellulosic Sugars. Ind. Crops Prod. 2014;52:158–168. doi: 10.1016/j.indcrop.2013.10.009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

