New Insights on the Uptake and Trafficking of Coenzyme Q
- PMID: 37507930
- PMCID: PMC10376127
- DOI: 10.3390/antiox12071391
New Insights on the Uptake and Trafficking of Coenzyme Q
Abstract
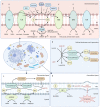
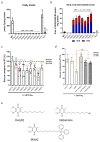
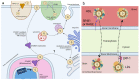
Coenzyme Q (CoQ) is an essential lipid with many cellular functions, such as electron transport for cellular respiration, antioxidant protection, redox homeostasis, and ferroptosis suppression. Deficiencies in CoQ due to aging, genetic disease, or medication can be ameliorated by high-dose supplementation. As such, an understanding of the uptake and transport of CoQ may inform methods of clinical use and identify how to better treat deficiency. Here, we review what is known about the cellular uptake and intracellular distribution of CoQ from yeast, mammalian cell culture, and rodent models, as well as its absorption at the organism level. We discuss the use of these model organisms to probe the mechanisms of uptake and distribution. The literature indicates that CoQ uptake and distribution are multifaceted processes likely to have redundancies in its transport, utilizing the endomembrane system and newly identified proteins that function as lipid transporters. Impairment of the trafficking of either endogenous or exogenous CoQ exerts profound effects on metabolism and stress response. This review also highlights significant gaps in our knowledge of how CoQ is distributed within the cell and suggests future directions of research to better understand this process.
Keywords: coenzyme Q; lipid trafficking; membrane contact sites; mitochondria transport; ubiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

