β-Asarone Alleviates High-Glucose-Induced Oxidative Damage via Inhibition of ROS Generation and Inactivation of the NF-κB/NLRP3 Inflammasome Pathway in Human Retinal Pigment Epithelial Cells
- PMID: 37507949
- PMCID: PMC10376195
- DOI: 10.3390/antiox12071410
β-Asarone Alleviates High-Glucose-Induced Oxidative Damage via Inhibition of ROS Generation and Inactivation of the NF-κB/NLRP3 Inflammasome Pathway in Human Retinal Pigment Epithelial Cells
Abstract
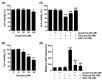
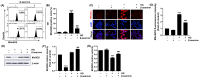
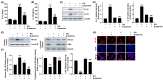
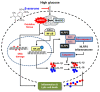
Diabetic retinopathy (DR) is the leading cause of vision loss and a major complication of diabetes. Hyperglycemia-induced accumulation of reactive oxygen species (ROS) is an important risk factor for DR. β-asarone, a major component of volatile oil extracted from Acori graminei Rhizoma, exerts antioxidant effects; however, its efficacy in DR remains unknown. In this study, we investigated whether β-asarone inhibits high-glucose (HG)-induced oxidative damage in human retinal pigment epithelial (RPE) ARPE-19 cells. We found that β-asarone significantly alleviated cytotoxicity, apoptosis, and DNA damage in HG-treated ARPE-19 cells via scavenging of ROS generation. β-Asarone also significantly attenuated the excessive accumulation of lactate dehydrogenase and mitochondrial ROS by increasing the manganese superoxide dismutase and glutathione activities. HG conditions markedly increased the release of interleukin (IL)-1β and IL-18 and upregulated their protein expression and activation of the nuclear factor-kappa B (NF-κB) signaling pathway, whereas β-asarone reversed these effects. Moreover, expression levels of the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome multiprotein complex molecules, including thioredoxin-interacting protein, NLRP3, apoptosis-associated speck-like protein containing a caspase-recruitment domain, and cysteinyl aspartate-specific proteinase-1, were increased in ARPE-19 cells under HG conditions. However, their expression levels remained similar to those in the control group in the presence of β-asarone. Therefore, β-asarone protects RPE cells from HG-induced injury by blocking ROS generation and NF-κB/NLRP3 inflammasome activation, indicating its potential as a therapeutic agent for DR treatment.
Keywords: NF-κB; NLRP3 inflammasome; ROS; high glucose; β-asarone.
Conflict of interest statement
The authors declare no conflict of interest. Each author of this study further declares no relationships with the companies or manufacturers who will benefit from the results of the present study.
Figures



Similar articles
-
Reduction of high glucose-induced oxidative injury in human retinal pigment epithelial cells by sarsasapogenin through inhibition of ROS generation and inactivation of NF-κB/NLRP3 inflammasome pathway.Genes Genomics. 2023 Sep;45(9):1153-1163. doi: 10.1007/s13258-023-01417-2. Epub 2023 Jun 24. Genes Genomics. 2023. PMID: 37354257
-
Spermidine Attenuates High Glucose-Induced Oxidative Damage in Retinal Pigment Epithelial Cells by Inhibiting Production of ROS and NF-κB/NLRP3 Inflammasome Pathway.Int J Mol Sci. 2023 Jun 23;24(13):10550. doi: 10.3390/ijms241310550. Int J Mol Sci. 2023. PMID: 37445726 Free PMC article.
-
Proanthocyanidins attenuate the high glucose-induced damage of retinal pigment epithelial cells by attenuating oxidative stress and inhibiting activation of the NLRP3 inflammasome.J Biochem Mol Toxicol. 2021 Sep;35(9):e22845. doi: 10.1002/jbt.22845. Epub 2021 Aug 2. J Biochem Mol Toxicol. 2021. PMID: 34338401
-
HDAC6 inhibitor Cay10603 inhibits high glucose-induced oxidative stress, inflammation and apoptosis in retinal pigment epithelial cells via regulating NF-κB and NLRP3 inflammasome pathway.Gen Physiol Biophys. 2020 Mar;39(2):169-177. doi: 10.4149/gpb_2019058. Gen Physiol Biophys. 2020. PMID: 32329444
-
[Curcumin alleviates nuclear factor-κB/NOD-like receptor protein 3 mediated renal injury caused by acute respiratory distress syndrome through reducing mitochondrial oxidative stress].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023 Apr;35(4):393-397. doi: 10.3760/cma.j.cn121430-20230414-00667. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023. PMID: 37308195 Chinese.
Cited by
-
Bupleuri Radix Ameliorates Vascular Inflammation in Human Umbilical Vein Endothelial Cells via Modulation of Tight Junction Protein Expression and Inhibition of Nuclear Factor-κB Activation.J Pharmacopuncture. 2025 Mar 31;28(1):57-68. doi: 10.3831/KPI.2025.28.1.57. J Pharmacopuncture. 2025. PMID: 40165875 Free PMC article.
-
High-Glucose-Induced Metabolic and Redox Alterations Are Distinctly Modulated by Various Antidiabetic Agents and Interventions Against FABP5/7, MITF and ANGPTL4 in Melanoma A375 Cells.Int J Mol Sci. 2025 Jan 24;26(3):1014. doi: 10.3390/ijms26031014. Int J Mol Sci. 2025. PMID: 39940783 Free PMC article.
-
Role of the NLRP3 inflammasome in diabetes and its complications (Review).Mol Med Rep. 2025 Nov;32(5):292. doi: 10.3892/mmr.2025.13657. Epub 2025 Aug 24. Mol Med Rep. 2025. PMID: 40849818 Free PMC article. Review.
-
Investigation of anti-diabetic effect of a novel coenzyme Q10 derivative.Front Chem. 2023 Oct 19;11:1280999. doi: 10.3389/fchem.2023.1280999. eCollection 2023. Front Chem. 2023. PMID: 37927560 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

