PrC-210 Protects against Radiation-Induced Hematopoietic and Intestinal Injury in Mice and Reduces Oxidative Stress
- PMID: 37507957
- PMCID: PMC10376632
- DOI: 10.3390/antiox12071417
PrC-210 Protects against Radiation-Induced Hematopoietic and Intestinal Injury in Mice and Reduces Oxidative Stress
Abstract
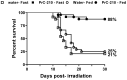
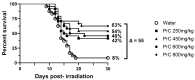
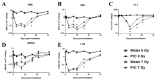
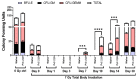
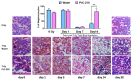
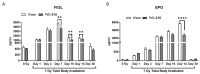
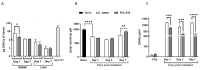
The development of safe, orally available, and effective prophylactic countermeasures to protect our warfighters is an unmet need because there is no such FDA-approved countermeasure available for use. Th 1-Propanethiol, 3-(methylamino)-2-((methylamino)methyl) (PrC-210), a synthetic small molecule, is a member of a new family of aminothiols designed to reduce toxicity while scavenging reactive oxygen species (ROS). Our study investigated the protective role of a single oral administration of PrC-210 against radiation-induced hematopoietic and intestinal injury in mice. Pre-treatment with PrC-210 significantly improved the survival of mice exposed to a lethal dose of radiation. Our findings indicated that the radioprotective properties of PrC-210 are achieved by accelerating the recovery of the hematopoietic system, stimulating bone marrow progenitor cells, and ameliorating additional biomarkers of hematopoietic injury. PrC-210 pre-treatment reduced intestinal injury in mice exposed to a lethal dose of radiation by restoring jejunal crypts and villi, reducing translocation of bacteria to the spleen, maintaining citrulline levels, and reducing the sepsis marker serum amyloid A (SAA) in serum. Finally, PrC-210 pre-treatment led to a significant reduction (~10 fold) of Nos2 expression (inducible nitric oxide) in the spleen and decreased oxidative stress by enhancing the antioxidant defense system. These data support the further development of PrC-210 to receive approval from the FDA to protect warfighters and first responders from exposure to the harmful effects of ionizing radiation.
Keywords: PrC-210; acute radiation syndrome; antioxidant; hematopoietic radiation injury; prophylactic countermeasure.
Conflict of interest statement
The following authors, V.P.K., S.B., G.H.H., and S.P.G., declare no conflict of interest. W.F. and T.G. are employees of Obvia Pharma and stand to benefit financially from the success of compound PrC-210.
Figures


Similar articles
-
Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice.Int J Mol Sci. 2018 May 21;19(5):1530. doi: 10.3390/ijms19051530. Int J Mol Sci. 2018. PMID: 29883417 Free PMC article.
-
Significant Reduction of Total-Body Irradiation-Induced Death in Mice Treated with PrC-210 24 Hours Postirradiation.Radiat Res. 2022 Sep 1;198(3):263-270. doi: 10.1667/RADE-22-00036.1. Radiat Res. 2022. PMID: 35728266
-
ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol.Radiat Res. 2012 Jul;178(1):57-68. doi: 10.1667/rr2806.1. Epub 2012 Jun 14. Radiat Res. 2012. PMID: 22702647 Free PMC article.
-
A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures.Int J Radiat Biol. 2017 Sep;93(9):851-869. doi: 10.1080/09553002.2017.1332438. Epub 2017 Jun 26. Int J Radiat Biol. 2017. PMID: 28650707 Review.
-
The efficacy and safety of amifostine for the acute radiation syndrome.Expert Opin Drug Saf. 2019 Nov;18(11):1077-1090. doi: 10.1080/14740338.2019.1666104. Epub 2019 Sep 17. Expert Opin Drug Saf. 2019. PMID: 31526195 Review.
Cited by
-
Significant Reduction of Radiation-Induced Death in Mice Treated with PrC-210 and G-CSF after Irradiation.Radiat Res. 2024 Oct 1;202(4):662-669. doi: 10.1667/RADE-24-00102.1. Radiat Res. 2024. PMID: 39142656
-
Human umbilical cord mesenchymal stem cell-derived exosomes mitigate acute radiation-induced intestinal oxidative damage via the Nrf2/HO-1/NQO1 signaling pathway.PLoS One. 2025 Jun 6;20(6):e0324238. doi: 10.1371/journal.pone.0324238. eCollection 2025. PLoS One. 2025. PMID: 40478886 Free PMC article.
References
-
- Epperly M.W., Bray J.A., Krager S., Berry L.M., Gooding W., Engelhardt J.F., Zwacka R., Travis E.L., Greenberger J.S. Intratracheal injection of adenovirus containing the human MNSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int. J. Radiat. Oncol. 1999;43:169–181. doi: 10.1016/S0360-3016(98)00355-1. - DOI - PubMed
-
- Kagan V.E., Bayır H.A., Belikova N.A., Kapralov O., Tyurina Y.Y., Tyurin V.A., Jiang J., Stoyanovsky D.A., Wipf P., Kochanek P.M., et al. Cytochrome c/cardiolipin relations in mito-chondria: A kiss of death. Free Radic. Biol. Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

