Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells
- PMID: 37507960
- PMCID: PMC10376204
- DOI: 10.3390/antiox12071422
Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells
Abstract
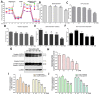
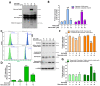
Osteosarcoma (OS) is the most common primary malignant bone tumor that produces immature osteoid. Metastatic OS has a poor prognosis with a death rate of >70%. Manoalide is a natural sesterterpenoid isolated from marine sponges. It is a phospholipase A2 inhibitor with anti-inflammatory, analgesic, and anti-cancer properties. This study aimed to investigate the mechanism and effect of manoalide on OS cells. Our experiments showed that manoalide induced cytotoxicity in 143B and MG63 cells (human osteosarcoma). Treatment with manoalide at concentrations of 10, 20, and 40 µM for 24 and 48 h reduced MG63 cell viability to 45.13-4.40% (p < 0.01). Meanwhile, manoalide caused reactive oxygen species (ROS) overproduction and disrupted antioxidant proteins, activating the apoptotic proteins caspase-9/-3 and PARP (Poly (ADP-ribose) polymerase). Excessive levels of ROS in the mitochondria affected oxidative phosphorylation, ATP generation, and membrane potential (ΔΨm). Additionally, manoalide down-regulated mitochondrial fusion protein and up-regulated mitochondrial fission protein, resulting in mitochondrial fragmentation and impaired function. On the contrary, a pre-treatment with n-acetyl-l-cysteine ameliorated manoalide-induced apoptosis, ROS, and antioxidant proteins in OS cells. Overall, our findings show that manoalide induces oxidative stress, mitochondrial dysfunction, and apoptosis, causing the cell death of OS cells, showing potential as an innovative alternative treatment in human OS.
Keywords: antioxidants; apoptosis; inflammation; mitochondria; mitochondrial respiratory chain; osteosarcoma; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Dilipde S.E., Paul J.S. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (polejaeff) Tetrahedron Lett. 1980;21:1611–1614. doi: 10.1016/S0040-4039(00)77766-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

