New NADPH Oxidase 2 Inhibitors Display Potent Activity against Oxidative Stress by Targeting p22phox-p47phox Interactions
- PMID: 37507978
- PMCID: PMC10376059
- DOI: 10.3390/antiox12071441
New NADPH Oxidase 2 Inhibitors Display Potent Activity against Oxidative Stress by Targeting p22phox-p47phox Interactions
Abstract
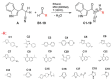
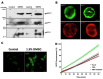
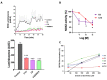
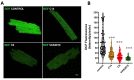
NADPH oxidase (NOX2) is responsible for reactive oxygen species (ROS) production in neutrophils and has been recognized as a key mediator in inflammatory and cardiovascular pathologies. Nevertheless, there is a lack of specific NOX2 pharmacological inhibitors. In medicinal chemistry, heterocyclic compounds are essential scaffolds for drug design, and among them, indole is a very versatile pharmacophore. We tested the hypothesis that indole heteroaryl-acrylonitrile derivatives may serve as NOX2 inhibitors by evaluating the capacity of 19 of these molecules to inhibit NOX2-derived ROS production in human neutrophils (HL-60 cells). Of these compounds, C6 and C14 exhibited concentration-dependent inhibition of NOX2 (IC50~1 µM). These molecules also reduced NOX2-derived oxidative stress in cardiomyocytes and prevented cardiac damage induced by ischemia-reperfusion. Compound C6 significantly reduced the membrane translocation of p47phox, a cytosolic subunit that is required for NOX2 activation. Molecular docking analyses of the binding modes of these molecules with p47phox indicated that C6 and C14 interact with specific residues in the inner part of the groove of p47phox, the binding cavity for p22phox. This combination of methods showed that novel indole heteroaryl acrylonitriles represent interesting lead compounds for developing specific and potent NOX2 inhibitors.
Keywords: HL-60 cells; NOX inhibitors; heteroaryl-acrylonitrile; mdx; p47phox; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
NOX2, p22phox and p47phox are targeted to the nuclear pore complex in ischemic cardiomyocytes colocalizing with local reactive oxygen species.Cell Physiol Biochem. 2011;27(5):471-8. doi: 10.1159/000329968. Epub 2011 Jun 15. Cell Physiol Biochem. 2011. PMID: 21691064
-
The protein kinase A negatively regulates reactive oxygen species production by phosphorylating gp91phox/NOX2 in human neutrophils.Free Radic Biol Med. 2020 Nov 20;160:19-27. doi: 10.1016/j.freeradbiomed.2020.07.021. Epub 2020 Aug 3. Free Radic Biol Med. 2020. PMID: 32758662
-
Stachydrine hydrochloride reduces NOX2 activity to suppress oxidative stress levels to improve cardiac insufficiency.Phytomedicine. 2025 May;140:156621. doi: 10.1016/j.phymed.2025.156621. Epub 2025 Mar 10. Phytomedicine. 2025. PMID: 40088741
-
NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12951. doi: 10.1111/eci.12951. Epub 2018 Jun 3. Eur J Clin Invest. 2018. PMID: 29757466 Review.
-
Arachidonic Acid and Nitroarachidonic: Effects on NADPH Oxidase Activity.Adv Exp Med Biol. 2019;1127:85-95. doi: 10.1007/978-3-030-11488-6_6. Adv Exp Med Biol. 2019. PMID: 31140173 Review.
Cited by
-
Structural profiles of the full phagocyte NADPH oxidase unveiled by combining computational biology and experimental knowledge.J Biol Chem. 2024 Dec;300(12):107943. doi: 10.1016/j.jbc.2024.107943. Epub 2024 Oct 29. J Biol Chem. 2024. PMID: 39481598 Free PMC article.
-
p47phox: A Central Regulator of NADPH Oxidase Function and a Promising Therapeutic Target in Redox-Related Diseases.Cells. 2025 Jul 8;14(14):1043. doi: 10.3390/cells14141043. Cells. 2025. PMID: 40710296 Free PMC article. Review.
-
Effects of tetrahydroindenoindole supplementation on metabolism: A systematic review with meta-analysis of rodent-based studies.Geroscience. 2025 May 5. doi: 10.1007/s11357-025-01680-z. Online ahead of print. Geroscience. 2025. PMID: 40323488
-
NADPH oxidases: redox regulation of cell homeostasis and disease.Physiol Rev. 2025 Jul 1;105(3):1291-1428. doi: 10.1152/physrev.00034.2023. Epub 2025 Jan 15. Physiol Rev. 2025. PMID: 39814410 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

