Quinones as Neuroprotective Agents
- PMID: 37508002
- PMCID: PMC10376830
- DOI: 10.3390/antiox12071464
Quinones as Neuroprotective Agents
Abstract
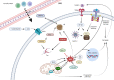
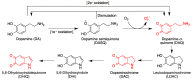
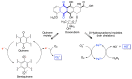
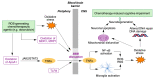
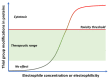
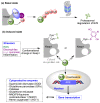
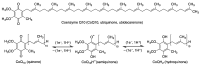
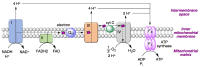
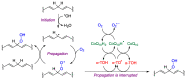
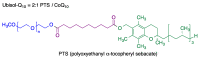
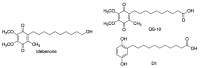
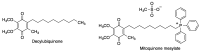
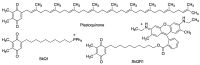
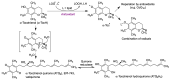
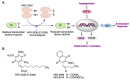
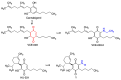
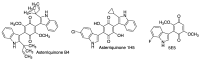
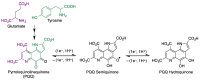
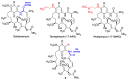
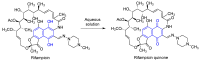
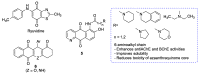
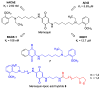
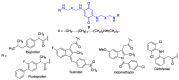
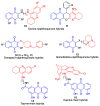
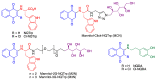
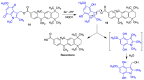
Quinones can in principle be viewed as a double-edged sword in the treatment of neurodegenerative diseases, since they are often cytoprotective but can also be cytotoxic due to covalent and redox modification of biomolecules. Nevertheless, low doses of moderately electrophilic quinones are generally cytoprotective, mainly due to their ability to activate the Keap1/Nrf2 pathway and thus induce the expression of detoxifying enzymes. Some natural quinones have relevant roles in important physiological processes. One of them is coenzyme Q10, which takes part in the oxidative phosphorylation processes involved in cell energy production, as a proton and electron carrier in the mitochondrial respiratory chain, and shows neuroprotective effects relevant to Alzheimer's and Parkinson's diseases. Additional neuroprotective quinones that can be regarded as coenzyme Q10 analogues are idobenone, mitoquinone and plastoquinone. Other endogenous quinones with neuroprotective activities include tocopherol-derived quinones, most notably vatiquinone, and vitamin K. A final group of non-endogenous quinones with neuroprotective activity is discussed, comprising embelin, APX-3330, cannabinoid-derived quinones, asterriquinones and other indolylquinones, pyrroloquinolinequinone and its analogues, geldanamycin and its analogues, rifampicin quinone, memoquin and a number of hybrid structures combining quinones with amino acids, cholinesterase inhibitors and non-steroidal anti-inflammatory drugs.
Keywords: Keap1/Nrf2 pathway; coenzyme Q10; covalent drugs; embelin; idebenone; memoquin; multitarget drugs; oxidative stress; pyrroloquinolinequinone; vatiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
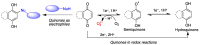

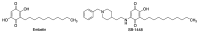


Similar articles
-
Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone.J Infect Public Health. 2020 Dec;13(12):1868-1877. doi: 10.1016/j.jiph.2020.09.015. Epub 2020 Oct 14. J Infect Public Health. 2020. PMID: 33109497 Free PMC article.
-
Quinones bearing non-steroidal anti-inflammatory fragments as multitarget ligands for Alzheimer's disease.Bioorg Med Chem Lett. 2013 Dec 1;23(23):6254-8. doi: 10.1016/j.bmcl.2013.09.091. Epub 2013 Oct 5. Bioorg Med Chem Lett. 2013. PMID: 24140444
-
NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels.PLoS One. 2011 Mar 31;6(3):e17963. doi: 10.1371/journal.pone.0017963. PLoS One. 2011. PMID: 21483849 Free PMC article.
-
The principal molecular mechanisms behind the activation of Keap1/Nrf2/ARE pathway leading to neuroprotective action in Parkinson's disease.Neurochem Int. 2022 Jun;156:105325. doi: 10.1016/j.neuint.2022.105325. Epub 2022 Mar 9. Neurochem Int. 2022. PMID: 35278519 Review.
-
Nonsteroidal anti-inflammatory drugs in Parkinson's disease: possible involvement of quinone formation.Expert Rev Neurother. 2006 Sep;6(9):1313-25. doi: 10.1586/14737175.6.9.1313. Expert Rev Neurother. 2006. PMID: 17009919 Review.
Cited by
-
Integrating a quinone substructure into histone deacetylase inhibitors to cope with Alzheimer's disease and cancer.RSC Med Chem. 2024 May 2;15(6):2045-2062. doi: 10.1039/d4md00175c. eCollection 2024 Jun 19. RSC Med Chem. 2024. PMID: 38911150 Free PMC article.
-
Complex roles for sulfation in the toxicities of polychlorinated biphenyls.Crit Rev Toxicol. 2024 Feb;54(2):92-122. doi: 10.1080/10408444.2024.2311270. Epub 2024 Feb 16. Crit Rev Toxicol. 2024. PMID: 38363552 Free PMC article. Review.
-
Insights into the Sources, Structure, and Action Mechanisms of Quinones on Diabetes: A Review.Molecules. 2025 Feb 3;30(3):665. doi: 10.3390/molecules30030665. Molecules. 2025. PMID: 39942768 Free PMC article. Review.
-
Multitarget Compounds Designed for Alzheimer, Parkinson, and Huntington Neurodegeneration Diseases.Pharmaceuticals (Basel). 2025 Jun 1;18(6):831. doi: 10.3390/ph18060831. Pharmaceuticals (Basel). 2025. PMID: 40573227 Free PMC article. Review.
-
Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles.Antioxidants (Basel). 2023 Jul 28;12(8):1511. doi: 10.3390/antiox12081511. Antioxidants (Basel). 2023. PMID: 37627506 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

