The 3Rs in Experimental Liver Disease
- PMID: 37508134
- PMCID: PMC10376896
- DOI: 10.3390/ani13142357
The 3Rs in Experimental Liver Disease
Abstract
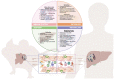
Patients with cirrhosis present multiple physiological and immunological alterations that play a very important role in the development of clinically relevant secondary complications to the disease. Experimentation in animal models is essential to understand the pathogenesis of human diseases and, considering the high prevalence of liver disease worldwide, to understand the pathophysiology of disease progression and the molecular pathways involved, due to the complexity of the liver as an organ and its relationship with the rest of the organism. However, today there is a growing awareness about the sensitivity and suffering of animals, causing opposition to animal research among a minority in society and some scientists, but also about the attention to the welfare of laboratory animals since this has been built into regulations in most nations that conduct animal research. In 1959, Russell and Burch published the book "The Principles of Humane Experimental Technique", proposing that in those experiments where animals were necessary, everything possible should be done to try to replace them with non-sentient alternatives, to reduce to a minimum their number, and to refine experiments that are essential so that they caused the least amount of pain and distress. In this review, a comprehensive summary of the most widely used techniques to replace, reduce, and refine in experimental liver research is offered, to assess the advantages and weaknesses of available experimental liver disease models for researchers who are planning to perform animal studies in the near future.
Keywords: disease; liver; reduction; refinement; replacement; research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

