Adsorption/Desorption of Cationic-Hydrophobic Peptides on Zwitterionic Lipid Bilayer Is Associated with the Possibility of Proton Transfer
- PMID: 37508312
- PMCID: PMC10376034
- DOI: 10.3390/antibiotics12071216
Adsorption/Desorption of Cationic-Hydrophobic Peptides on Zwitterionic Lipid Bilayer Is Associated with the Possibility of Proton Transfer
Abstract
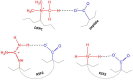
Cell-penetrating peptides (CPPs) are short peptides built up from dominantly cationic and hydrophobic amino acid residues with a distinguished ability to pass through the cell membrane. Due to the possibility of linking and delivering the appropriate cargo at the desired location, CPPs are considered an economic and less invasive alternative to antibiotics. Besides knowing that their membrane passage mechanism is a complex function of CPP chemical composition, the ionic strength of the solution, and the membrane composition, all other details on how they penetrate cell membranes are rather vague. The aim of this study is to elucidate the ad(de)sorption of arginine-/lysine- and phenylalanine-rich peptides on a lipid membrane composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) lipids. DSC and temperature-dependent UV-Vis measurements confirmed the impact of the adsorbed peptides on thermotropic properties of DPPC, but in an inconclusive way. On the other hand, FTIR spectra acquired at 30 °C and 50 °C (when DPPC lipids are found in the gel and fluid phase, respectively) unambiguously confirmed the proton transfer between particular titratable functional groups of R5F2/K5F2 that highly depend on their immediate surroundings (DPPC or a phosphate buffer). Molecular dynamic simulations showed that both peptides may adsorb onto the bilayer, but K5F2 desorbs more easily and favors the solvent, while R5F2 remains attached. The results obtained in this work highlight the importance of proton transfer in the design of CPPs with their desired cargo, as its charge and composition dictates the possibility of entering the cell.
Keywords: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); FTIR and UV-Vis spectroscopy; MD simulations; adsorption mechanism; cell-penetrating peptides R5F2 and K5F2; large unilamellar liposomes (LUV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Phase-Dependent Adsorption of Myelin Basic Protein to Phosphatidylcholine Lipid Bilayers.Membranes (Basel). 2024 Jan 4;14(1):15. doi: 10.3390/membranes14010015. Membranes (Basel). 2024. PMID: 38248705 Free PMC article.
-
Interaction of guanidinium and ammonium cations with phosphatidylcholine and phosphatidylserine lipid bilayers - Calorimetric, spectroscopic and molecular dynamics simulations study.Biochim Biophys Acta Biomembr. 2023 Apr;1865(4):184122. doi: 10.1016/j.bbamem.2023.184122. Epub 2023 Feb 3. Biochim Biophys Acta Biomembr. 2023. PMID: 36739930
-
The helical propensity of KLA amphipathic peptides enhances their binding to gel-state lipid membranes.Biophys Chem. 2013 Oct-Nov;180-181:10-21. doi: 10.1016/j.bpc.2013.05.003. Epub 2013 May 24. Biophys Chem. 2013. PMID: 23792704
-
Solution NMR studies of cell-penetrating peptides in model membrane systems.Adv Drug Deliv Rev. 2013 Jul;65(8):1002-11. doi: 10.1016/j.addr.2012.10.011. Epub 2012 Nov 6. Adv Drug Deliv Rev. 2013. PMID: 23137785 Review.
-
How Membrane-Active Peptides Get into Lipid Membranes.Acc Chem Res. 2016 Jun 21;49(6):1130-8. doi: 10.1021/acs.accounts.6b00074. Epub 2016 May 17. Acc Chem Res. 2016. PMID: 27187572 Review.
Cited by
-
Biophysical Insights into the Antitumoral Activity of Crotalicidin against Breast Cancer Model Membranes.Int J Mol Sci. 2023 Nov 12;24(22):16226. doi: 10.3390/ijms242216226. Int J Mol Sci. 2023. PMID: 38003414 Free PMC article.
References
-
- Ancillotti M., Eriksson S., Andersson D.I., Godskesen T., Nihlén Fahlquist J., Veldwijk J. Preferences Regarding Antibiotic Treatment and the Role of Antibiotic Resistance: A Discrete Choice Experiment. Int. J. Antimicrob. Agents. 2020;56:106198. doi: 10.1016/j.ijantimicag.2020.106198. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

