Combination Effects of Integrin-linked Kinase and Abelson Kinase Inhibition on Aberrant Mitosis and Cell Death in Glioblastoma Cells
- PMID: 37508338
- PMCID: PMC10376030
- DOI: 10.3390/biology12070906
Combination Effects of Integrin-linked Kinase and Abelson Kinase Inhibition on Aberrant Mitosis and Cell Death in Glioblastoma Cells
Abstract
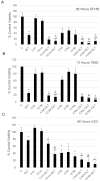
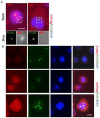
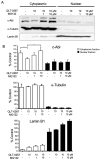
In cancer cells, inhibition of integrin-linked kinase (ILK) increases centrosome declustering causing mitotic arrest and cell death. Yet, not all cancer cells are susceptible to anti-ILK treatment alone. We investigate a combination drug strategy targeting ILK and another oncogenic kinase, Abelson kinase (ABL). Drug-concentration viability assays (i.e., MTT assays) indicate that ILK and ABL inhibitors in combination decreased the viability of glioblastoma cells over the ILK drug QLT-0267 alone. Combination strategies also increased aberrant mitoses and cell death over QLT-0267 alone. This was evident from an increase in mitotic arrest, apoptosis and a sub-G1 peak following FAC analysis. In vitro, ILK and ABL localized to the centrosome and the putative ILK kinase domain was important for this localization. Increased levels of cytosolic ABL are associated with its transformative abilities. ILK inhibitor effects on survival correlated with its ability to decrease cytosolic ABL levels and inhibit ABL's localization to mitotic centrosomes in glioblastoma cells. ILK inhibitor effects on ABL's centrosomal localization were reversed by the proteasomal inhibitor MG132 (a drug that inhibits ABL degradation). These results indicate that ILK regulates ABL at mitotic centrosomes and that combination treatments targeting ILK and ABL are more effective then QLT-0267 alone at decreasing the survival of dividing glioblastoma cells.
Keywords: Abelson kinase; apoptosis; centrosome declustering; glioblastoma; integrin-linked kinase; mitotic arrest.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Edwards L.A., Woo J., Huxham L.A., Verreault M., Dragowska W.H., Chiu G., Rajput A., Kyle A.H., Kalra J., Yapp D., et al. Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK) Mol. Cancer Ther. 2008;7:59–70. doi: 10.1158/1535-7163.MCT-07-0329. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

