Proline Isomerization: From the Chemistry and Biology to Therapeutic Opportunities
- PMID: 37508437
- PMCID: PMC10376262
- DOI: 10.3390/biology12071008
Proline Isomerization: From the Chemistry and Biology to Therapeutic Opportunities
Abstract
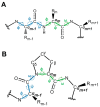
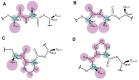
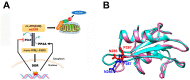
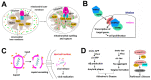
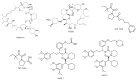
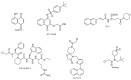
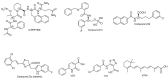
Proline isomerization, the process of interconversion between the cis- and trans-forms of proline, is an important and unique post-translational modification that can affect protein folding and conformations, and ultimately regulate protein functions and biological pathways. Although impactful, the importance and prevalence of proline isomerization as a regulation mechanism in biological systems have not been fully understood or recognized. Aiming to fill gaps and bring new awareness, we attempt to provide a wholistic review on proline isomerization that firstly covers what proline isomerization is and the basic chemistry behind it. In this section, we vividly show that the cause of the unique ability of proline to adopt both cis- and trans-conformations in significant abundance is rooted from the steric hindrance of these two forms being similar, which is different from that in linear residues. We then discuss how proline isomerization was discovered historically followed by an introduction to all three types of proline isomerases and how proline isomerization plays a role in various cellular responses, such as cell cycle regulation, DNA damage repair, T-cell activation, and ion channel gating. We then explore various human diseases that have been linked to the dysregulation of proline isomerization. Finally, we wrap up with the current stage of various inhibitors developed to target proline isomerases as a strategy for therapeutic development.
Keywords: 5-hydroxytryptamine type 3 (5-HT3); Alzheimer’s disease (AD); Ataxia telangiectasia and Rad3-related (ATR); FK506 binding protein (FKBP); Interleukin-2 inducible T-cell kinase (Itk); Parkinson’s disease (PD); Protein Interactor with NIMA1 (Pin1); autoimmune disease; cancer; cyclophilin; cyclosporin; hepatitis B virus (HBV); hepatitis C virus (HCV); human immunodeficiency virus 1 (HIV-1); infectious disease; multiple sclerosis (MS); neurodegenerative disease; parvulin; post-translation modifications; proline isomerase; proline isomerization; sanglifehrin; systemic lupus erythematosus (SLE).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Willstatter R. Synthesis of hygric acid. Berichte Dtsch. Chem. Ges. 1900;33:1160. doi: 10.1002/cber.190003301201. - DOI
-
- Fischer E. Hydrolysis of casein by means of hydrochloric acid. J. Chem. Soc. 1901;33:151–176.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

