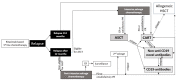
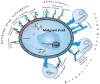
Redefining Precision Management of r/r Large B-Cell Lymphoma: Novel Antibodies Take on CART and BMT in the Quest for Future Treatment Strategies
- PMID: 37508523
- PMCID: PMC10378108
- DOI: 10.3390/cells12141858
Redefining Precision Management of r/r Large B-Cell Lymphoma: Novel Antibodies Take on CART and BMT in the Quest for Future Treatment Strategies
Abstract
The treatment paradigms for patients with relapsed large B-cell lymphoma are expanding. Chimeric antigen receptor technology (CAR-T) has revolutionized the management of these patients. Novel bispecific antibodies and antibody-drug conjugates, used as chemotherapy-free single agents or in combination with other novel therapeutics, have been quickly introduced into the real-world setting. With such a paradigm shift, patients have an improved chance of better outcomes with unpredictable complete remission rates. Additionally, the excellent tolerance of new antibodies targeting B-cell lymphomas is another motivation to broaden its use in relapsed and refractory patients. With the increasing number of approved therapy approaches, future research needs to focus on optimizing the sequence and developing new combination strategies for these antibodies, both among themselves and with other agents. Clinical, pathological, and genetic risk profiling can assist in identifying which patients are most likely to benefit from these costly therapeutic options. However, new combinations may lead to new side effects, which we must learn to deal with. This review provides a comprehensive overview of the current state of research on several innovative antibodies for the precision management of large B-cell lymphoma. It explores various treatment strategies, such as CAR-T vs. ASCT, naked antibodies, antibody-drug conjugates, bispecific antibodies, and bispecific T-cell engagers, as well as discussing the challenges and future perspectives of novel treatment strategies. We also delve into resistance mechanisms and factors that may affect decision making. Moreover, each section provides a detailed analysis of the available literature and ongoing clinical trials.
Keywords: CART; LBL; antibody–drug conjugate; bispecific antibodies; large B-cell lymphoma.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Li W. Leukemia. Exon Publications; Brisbane, Australia: 2022. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. Chapter 1. - PubMed
-
- Kanas G., Ge W., Quek R.G.W., Keeven K., Nersesyan K., Arnason J.E.A. Epidemiology of Diffuse Large B-Cell Lymphoma (DLBCL) and Follicular Lymphoma (FL) in the United States and Western Europe: Population-Level Projections for 2020–2025. Leuk. Lymphoma. 2022;63:54–63. doi: 10.1080/10428194.2021.1975188. - DOI - PubMed
-
- Epperla N., Vaughn J.L., Othus M., Hallack A., Costa L.J. CHOP-like Chemotherapy plus Rituximab versus CHOP-like Chemotherapy Alone in Young Patients with Good-Prognosis Diffuse Large-B-Cell Lymphoma: A Randomised Controlled Trial by the MabThera International Trial (MInT) Group. Cancer Med. 2020;9:5519–5525. doi: 10.1002/cam4.3237. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources