Macromolecule Translocation across the Intestinal Mucosa of HIV-Infected Patients by Transcytosis and through Apoptotic Leaks
- PMID: 37508551
- PMCID: PMC10378197
- DOI: 10.3390/cells12141887
Macromolecule Translocation across the Intestinal Mucosa of HIV-Infected Patients by Transcytosis and through Apoptotic Leaks
Abstract
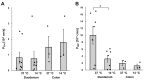
Based on indirect evidence, increased mucosal translocation of gut-derived microbial macromolecules has been proposed as an important pathomechanism in HIV infection. Here, we quantified macromolecule translocation across intestinal mucosa from treatment-naive HIV-infected patients, HIV-infected patients treated by combination antiretroviral therapy, and HIV-negative controls and analyzed the translocation pathways involved. Macromolecule permeability was quantified by FITC-Dextran 4000 (FD4) and horseradish peroxidase (HRP) flux measurements. Translocation pathways were addressed using cold inhibition experiments. Tight junction proteins were characterized by immunoblotting. Epithelial apoptosis was quantified and translocation pathways were further characterized by flux studies in T84 cell monolayers using inducers and inhibitors of apoptosis and endocytosis. In duodenal mucosa of untreated but not treated HIV-infected patients, FD4 and HRP permeabilities were more than a 4-fold increase compared to the HIV-negative controls. Duodenal macromolecule permeability was partially temperature-dependent and associated with epithelial apoptosis without altered expression of the analyzed tight junction proteins. In T84 monolayers, apoptosis induction increased, and both apoptosis and endocytosis inhibitors reduced macromolecule permeability. Using quantitative analysis, we demonstrate the increased macromolecule permeability of the intestinal mucosa in untreated HIV-infected patients. Combining structural and mechanistic studies, we identified two pathways of increased macromolecule translocation in HIV infection: transcytosis and passage through apoptotic leaks.
Keywords: HIV; apoptosis; intestine; macromolecule passage; tight junction; transcytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ancuta P., Kamat A., Kunstman K.J., Kim E.Y., Autissier P., Wurcel A., Zaman T., Stone D., Mefford M., Morgello S., et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. - DOI - PMC - PubMed
-
- Marchetti G., Bellistri G.M., Borghi E., Tincati C., Ferramosca S., La Francesca M., Morace G., Gori A., Monforte A.D. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. - DOI - PubMed
-
- Cassol E., Malfeld S., Mahasha P., van der Merwe S., Cassol S., Seebregts C., Alfano M., Poli G., Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J. Infect. Dis. 2010;202:723–733. doi: 10.1086/655229. - DOI - PubMed
-
- Jiang W., Lederman M.M., Hunt P., Sieg S.F., Haley K., Rodriguez B., Landay A., Martin J., Sinclair E., Asher A.I., et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 2009;199:1177–1185. doi: 10.1086/597476. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

