Integrated Collection of Patient-Reported Outcomes and Experiences in Children with Kidney and Hematological Diseases: A Pilot Study
- PMID: 37508742
- PMCID: PMC10377830
- DOI: 10.3390/children10071245
Integrated Collection of Patient-Reported Outcomes and Experiences in Children with Kidney and Hematological Diseases: A Pilot Study
Abstract
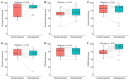
We aimed to explore the feasibility and potential relevance of integrated electronic collection of patient-reported outcome and experience measures (PROM and PREM) in children with special healthcare needs (CSHCN) by using the example of children with kidney and hematological diseases. We performed a cross-sectional, single-center study of children <18 years of age. Children (≥10 years) and their parents received Generic PedsQL Core Scale 4.0 and custom PREM surveys to their email addresses via the REDCap platform, and the results were integrated into the hospital's electronic health records system. A total of 192 patients (98 with kidney diseases and 94 with hematological diseases) were enrolled. The overall response rate was 51%, and the median time for completion of each proxy questionnaire was approximately three minutes. The lowest PROM scores were observed in the emotional and school functioning dimensions. More favorable experiences in the diagnosis establishment process were associated with higher scores in physical, social, school functioning, and total PROM scores. A better evaluation of the hospital's environment was associated with higher social functioning, while better information provision correlated with higher physical functioning and total PROM scores. Our data indicates that integrated electronic collection of PROMs and PREMs in the population of CSHCN is feasible, but efforts to increase the response rate are needed. The associations between PROMs and PREMs suggest that future studies exploring targeted interventions at the healthcare service level to improve subjective patient outcomes are needed.
Keywords: PREM; PROM; children; hematological disease; kidney disease; parents; patient-reported experience; patient-reported outcome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






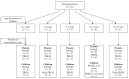
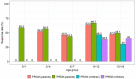


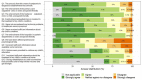
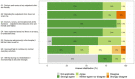
Similar articles
-
Development and Integration of Patient-Reported Measures into E-Health System: Pilot Feasibility Study.Healthcare (Basel). 2023 Aug 14;11(16):2290. doi: 10.3390/healthcare11162290. Healthcare (Basel). 2023. PMID: 37628488 Free PMC article.
-
Requirements for the collection of electronic PROMS either "in clinic" or "at home" as part of the PROMs, PREMs and Effectiveness Programme (PPEP) in Wales: a feasibility study using a generic PROM tool.Pilot Feasibility Stud. 2018 Jul 4;4:90. doi: 10.1186/s40814-018-0282-8. eCollection 2018. Pilot Feasibility Stud. 2018. PMID: 29997899 Free PMC article.
-
PROMs and PREMs in routine perinatal care: mixed methods evaluation of their implementation into integrated obstetric care networks.J Patient Rep Outcomes. 2023 Mar 9;7(1):26. doi: 10.1186/s41687-023-00568-w. J Patient Rep Outcomes. 2023. PMID: 36894797 Free PMC article.
-
Implementation of patient-reported outcome measures and patient-reported experience measures in melanoma clinical quality registries: a systematic review.BMJ Open. 2021 Feb 11;11(2):e040751. doi: 10.1136/bmjopen-2020-040751. BMJ Open. 2021. PMID: 33574144 Free PMC article.
-
Use of patient-reported experience and outcome measures within the colorectal cancer care continuum: a scoping review.J Cancer Surviv. 2024 Apr 16. doi: 10.1007/s11764-024-01595-2. Online ahead of print. J Cancer Surviv. 2024. PMID: 38627293 Review.
References
-
- Kingsley C., Patel S. Patient-Reported Outcome Measures and Patient-Reported Experience Measures. BJA Educ. 2017;17:137–144. doi: 10.1093/bjaed/mkw060. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

