Co-Electrospun Poly(ε-Caprolactone)/Zein Articular Cartilage Scaffolds
- PMID: 37508797
- PMCID: PMC10376865
- DOI: 10.3390/bioengineering10070771
Co-Electrospun Poly(ε-Caprolactone)/Zein Articular Cartilage Scaffolds
Abstract
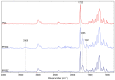
Osteoarthritis scaffold-based grafts fail because of poor integration with the surrounding soft tissue and inadequate tribological properties. To circumvent this, we propose electrospun poly(ε-caprolactone)/zein-based scaffolds owing to their biomimetic capabilities. The scaffold surfaces were characterized using Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy, static water contact angles, and profilometry. Scaffold biocompatibility properties were assessed by measuring protein adsorption (Bicinchoninic Acid Assay), cell spreading (stained F-actin), and metabolic activity (PrestoBlue™ Cell Viability Reagent) of primary bovine chondrocytes. The data show that zein surface segregation in the membranes not only completely changed the hydrophobic behavior of the materials, but also increased the cell yield and metabolic activity on the scaffolds. The surface segregation is verified by the infrared peak at 1658 cm-1, along with the presence and increase in N1 content in the survey XPS. This observation could explain the decrease in the water contact angles from 125° to approximately 60° in zein-comprised materials and the decrease in the protein adsorption of both bovine serum albumin and synovial fluid by half. Surface nano roughness in the PCL/zein samples additionally benefited the radial spreading of bovine chondrocytes. This study showed that co-electrospun PCL/zein scaffolds have promising surface and biocompatibility properties for use in articular-tissue-engineering applications.
Keywords: PCL; cartilage tissue engineering; chondrocytes; electrospinning; zein.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- O’Brien F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

