Model-Based Characterization of E. coli Strains with Impaired Glucose Uptake
- PMID: 37508835
- PMCID: PMC10376147
- DOI: 10.3390/bioengineering10070808
Model-Based Characterization of E. coli Strains with Impaired Glucose Uptake
Abstract
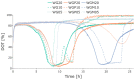
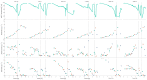
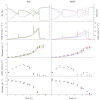
The bacterium Escherichia coli is a widely used organism in biotechnology. For high space-time yields, glucose-limited fed-batch technology is the industry standard; this is because an overflow metabolism of acetate occurs at high glucose concentrations. As an interesting alternative, various strains with limited glucose uptake have been developed. However, these have not yet been characterized under process conditions. To demonstrate the efficiency of our previously developed high-throughput robotic platform, in the present work, we characterized three different exemplary E. coli knockout (KO) strains with limited glucose uptake capacities at three different scales (microtiter plates, 10 mL bioreactor system and 100 mL bioreactor system) under excess glucose conditions with different initial glucose concentrations. The extensive measurements of growth behavior, substrate consumption, respiration, and overflow metabolism were then used to determine the appropriate growth parameters using a mechanistic mathematical model, which allowed for a comprehensive comparative analysis of the strains. The analysis was performed coherently with these different reactor configurations and the results could be successfully transferred from one platform to another. Single and double KO mutants showed reduced specific rates for substrate uptake qSmax and acetate production qApmax; meanwhile, higher glucose concentrations had adverse effects on the biomass yield coefficient YXSem. Additional parameters compared to previous studies for the oxygen uptake rate and carbon dioxide production rate indicated differences in the specific oxygen uptake rate qOmax. This study is an example of how automated robotic equipment, together with mathematical model-based approaches, can be successfully used to characterize strains and obtain comprehensive information more quickly, with a trade-off between throughput and analytical capacity.
Keywords: high throughput; laboratory automation; model-based; strain characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Neubauer P., Cruz Bournazou M.N., Glauche F., Junne S., Knepper A., Raven M. Consistent development of bioprocesses from microliter cultures to the industrial scale. Eng. Life Sci. 2013;13:224–238. doi: 10.1002/elsc.201200021. - DOI
-
- Pan J.G., Rhee J.S., Lebeault J.M. Physiological constraints in increasing biomass concentration of Escherichia coli B in fed-batch culture. Biotechnol. Lett. 1987;9:89–94. doi: 10.1007/BF01032744. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

