Characterization of Mechanical and Cellular Effects of Rhythmic Vertical Vibrations on Adherent Cell Cultures
- PMID: 37508838
- PMCID: PMC10376548
- DOI: 10.3390/bioengineering10070811
Characterization of Mechanical and Cellular Effects of Rhythmic Vertical Vibrations on Adherent Cell Cultures
Abstract
This paper presents an innovative experimental setup that employs the principles of audio technology to subject adherent cells to rhythmic vertical vibrations. We employ a novel approach that combines three-axis acceleration measurements and particle tracking velocimetry to evaluate the setup's performance. This allows us to estimate crucial parameters such as root mean square acceleration, fluid flow patterns, and shear stress generated within the cell culture wells when subjected to various vibration types. The experimental conditions consisted of four vibrational modes: No Vibration, Continuous Vibration, Regular Pulse, and Variable Pulse. To evaluate the effects on cells, we utilized fluorescence microscopy and a customized feature extraction algorithm to analyze the F-actin filament structures. Our findings indicate a consistent trend across all vibrated cell cultures, revealing a reduction in size and altered orientation (2D angle) of the filaments. Furthermore, we observed cell accumulations in the G1 cell cycle phase in cells treated with Continuous Vibration and Regular Pulse. Our results demonstrate a negative correlation between the magnitude of mechanical stimuli and the size of F-actin filaments, as well as a positive correlation with the accumulations of cells in the G1 phase of the cell cycle. By unraveling these analyses, this study paves the way for future investigations and provides a compelling framework for comprehending the intricate cellular responses to rhythmic mechanical stimulation.
Keywords: F-actin filament angle; F-actin filament length; F-actin filament thickness; Max/MSP signal generation; acceleration; image feature extraction; mechanobiology; particle tracking velocimetry (PTV); rhythmic vertical vibration; shear stress; sound vibrations.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
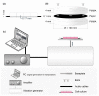

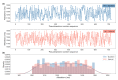



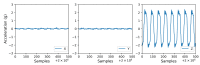


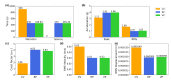





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

