Perfusion of MC3T3E1 Preosteoblast Spheroids within Polysaccharide-Based Hydrogel Scaffolds: An Experimental and Numerical Study at the Bioreactor Scale
- PMID: 37508876
- PMCID: PMC10376891
- DOI: 10.3390/bioengineering10070849
Perfusion of MC3T3E1 Preosteoblast Spheroids within Polysaccharide-Based Hydrogel Scaffolds: An Experimental and Numerical Study at the Bioreactor Scale
Abstract
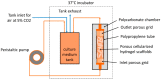
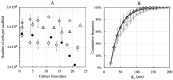
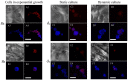
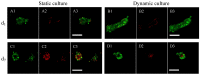
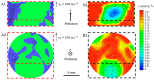
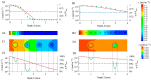
The traditional 3D culture systems in vitro lack the biological and mechanical spatiotemporal stimuli characteristic to native tissue development. In our study, we combined porous polysaccharide-based hydrogel scaffolds with a bioreactor-type perfusion device that generates favorable mechanical stresses while enhancing nutrient transfers. MC3T3E1 mouse osteoblasts were seeded in the scaffolds and cultivated for 3 weeks under dynamic conditions at a perfusion rate of 10 mL min-1. The spatial distribution of the cells labeled with superparamagnetic iron oxide nanoparticles was visualized by MRI. Confocal microscopy was used to assess cell numbers, their distribution inside the scaffolds, cell viability, and proliferation. The oxygen diffusion coefficient in the hydrogel was measured experimentally. Numerical simulations of the flow and oxygen transport within the bioreactor were performed using a lattice Boltzmann method with a two-relaxation time scheme. Last, the influence of cell density and spheroid size on cell oxygenation was investigated. The cells spontaneously organized into spheroids with a diameter of 30-100 μm. Cell viability remained unchanged under dynamic conditions but decreased under static culture. The cell proliferation (Ki67 expression) in spheroids was not observed. The flow simulation showed that the local fluid velocity reached 27 mm s-1 at the height where the cross-sectional area of the flow was the smallest. The shear stress exerted by the fluid on the scaffolds may locally rise to 100 mPa, compared with the average value of 25 mPa. The oxygen diffusion coefficient in the hydrogel was 1.6×10-9 m2 s-1. The simulation of oxygen transport and consumption confirmed that the cells in spheroids did not suffer from hypoxia when the bioreactor was perfused at 10 mL min-1, and suggested the existence of optimal spheroid size and spacing for appropriate oxygenation. Collectively, these findings enabled us to define the optimal conditions inside the bioreactor for an efficient in vitro cell organization and survival in spheroids, which are paramount to future applications with organoids.
Keywords: 3D cell culture; fluid dynamics; lattice Boltzmann method; organoids; oxygen transport; perfusion bioreactor; porous hydrogel; spheroids.
Conflict of interest statement
Didier Letourneur has shares in SILTISS Company which holds four patents on these scaffolds for tissue engineering.
Figures





Similar articles
-
Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite.Gels. 2024 Oct 18;10(10):666. doi: 10.3390/gels10100666. Gels. 2024. PMID: 39451319 Free PMC article.
-
Inlet flow rate of perfusion bioreactors affects fluid flow dynamics, but not oxygen concentration in 3D-printed scaffolds for bone tissue engineering: Computational analysis and experimental validation.Comput Biol Med. 2020 Sep;124:103826. doi: 10.1016/j.compbiomed.2020.103826. Epub 2020 Aug 4. Comput Biol Med. 2020. PMID: 32798924
-
Numerical Investigations of Hepatic Spheroids Metabolic Reactions in a Perfusion Bioreactor.Front Bioeng Biotechnol. 2019 Sep 12;7:221. doi: 10.3389/fbioe.2019.00221. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31572719 Free PMC article.
-
3-D computational modeling of media flow through scaffolds in a perfusion bioreactor.J Biomech. 2005 Mar;38(3):543-9. doi: 10.1016/j.jbiomech.2004.04.011. J Biomech. 2005. PMID: 15652553
-
Modulation of cell differentiation in bone tissue engineering constructs cultured in a bioreactor.Adv Exp Med Biol. 2006;585:225-41. doi: 10.1007/978-0-387-34133-0_16. Adv Exp Med Biol. 2006. PMID: 17120788 Review.
Cited by
-
In vitro and in vivo assessment of a non-animal sourced chitosan scaffold loaded with xeno-free umbilical cord mesenchymal stromal cells cultured under macromolecular crowding conditions.Biomater Biosyst. 2024 Oct 10;16:100102. doi: 10.1016/j.bbiosy.2024.100102. eCollection 2024 Dec. Biomater Biosyst. 2024. PMID: 40225717 Free PMC article.
-
Cancer-on-a-chip for precision cancer medicine.Lab Chip. 2025 Jul 8;25(14):3314-3347. doi: 10.1039/d4lc01043d. Lab Chip. 2025. PMID: 40376718 Free PMC article. Review.
-
Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations.Bioengineering (Basel). 2023 Oct 28;10(11):1257. doi: 10.3390/bioengineering10111257. Bioengineering (Basel). 2023. PMID: 38002381 Free PMC article.
-
Bone Spheroid Development Under Flow Conditions with Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in a 3D Porous Hydrogel Supplemented with Hydroxyapatite.Gels. 2024 Oct 18;10(10):666. doi: 10.3390/gels10100666. Gels. 2024. PMID: 39451319 Free PMC article.
-
Biomimetic Design and Assessment via Microenvironmental Testing: From Food Packaging Biomaterials to Implantable Medical Devices.Biomimetics (Basel). 2025 Jun 5;10(6):370. doi: 10.3390/biomimetics10060370. Biomimetics (Basel). 2025. PMID: 40558339 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

