Assessing Tumorigenicity in Stem Cell-Derived Therapeutic Products: A Critical Step in Safeguarding Regenerative Medicine
- PMID: 37508884
- PMCID: PMC10376867
- DOI: 10.3390/bioengineering10070857
Assessing Tumorigenicity in Stem Cell-Derived Therapeutic Products: A Critical Step in Safeguarding Regenerative Medicine
Abstract
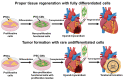
Stem cells hold promise in regenerative medicine due to their ability to proliferate and differentiate into various cell types. However, their self-renewal and multipotency also raise concerns about their tumorigenicity during and post-therapy. Indeed, multiple studies have reported the presence of stem cell-derived tumors in animal models and clinical administrations. Therefore, the assessment of tumorigenicity is crucial in evaluating the safety of stem cell-derived therapeutic products. Ideally, the assessment needs to be performed rapidly, sensitively, cost-effectively, and scalable. This article reviews various approaches for assessing tumorigenicity, including animal models, soft agar culture, PCR, flow cytometry, and microfluidics. Each method has its advantages and limitations. The selection of the assay depends on the specific needs of the study and the stage of development of the stem cell-derived therapeutic product. Combining multiple assays may provide a more comprehensive evaluation of tumorigenicity. Future developments should focus on the optimization and standardization of microfluidics-based methods, as well as the integration of multiple assays into a single platform for efficient and comprehensive evaluation of tumorigenicity.
Keywords: cell therapy; regenerative medicine; stem cells; tumorigenicity.
Conflict of interest statement
Z.W. is a paid consultant of CTRL Therapeutics, a company utilizing magnetic cytometry for developing cellular therapy.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

