In Vivo Assessment of High-Strength and Corrosion-Controlled Magnesium-Based Bone Implants
- PMID: 37508904
- PMCID: PMC10376803
- DOI: 10.3390/bioengineering10070877
In Vivo Assessment of High-Strength and Corrosion-Controlled Magnesium-Based Bone Implants
Abstract
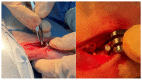
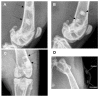
The biodegradable nature of magnesium in aqueous mediums makes it an attractive material for various biomedical applications when it is not recommended that the material stay permanently in the body. Some of the main challenges that hinder the use of magnesium for bone fracture repair are its limited mechanical strength and fast corrosion rates. To this end, we developed a novel Mg-Zn-Ca-Mn-based alloy and post-fabrication methods that can deliver high-strength and corrosion-controlled implant materials to address these challenges. This study is focused on assessing the in vitro corrosion and in vivo biocompatibility of the developed magnesium-based alloy and post-fabrication processes. The developed heat treatment process resulted in an increase in the microhardness from 71.9 ± 5.4 HV for the as-cast Mg alloy to as high as 98.1 ± 6.5 HV for the heat-treated Mg alloy, and the ceramic coating resulted in a significant reduction in the corrosion rate from 10.37 mm/yr for the uncoated alloy to 0.03 mm/yr after coating. The in vivo assessments showed positive levels of biocompatibility in terms of degradation rates and integration of the implants in a rabbit model. In the rabbit studies, the implants became integrated into the bone defect and showed minimal evidence of an immune response. The results of this study show that it is possible to produce biocompatible Mg-based implants with stronger and more corrosion-controlled properties based on the developed Mg-Zn-Ca-Mn-based alloy and post-fabrication methods.
Keywords: biodegradable; bone implants; in vitro; in vivo; magnesium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Microstructural, mechanical and corrosion characteristics of heat-treated Mg-1.2Zn-0.5Ca (wt%) alloy for use as resorbable bone fixation material.J Mech Behav Biomed Mater. 2017 May;69:203-212. doi: 10.1016/j.jmbbm.2017.01.005. Epub 2017 Jan 5. J Mech Behav Biomed Mater. 2017. PMID: 28088072
-
In vitro and in vivo assessment of squeeze-cast Mg-Zn-Ca-Mn alloys for biomedical applications.Acta Biomater. 2022 Sep 15;150:442-455. doi: 10.1016/j.actbio.2022.07.040. Epub 2022 Jul 29. Acta Biomater. 2022. PMID: 35914693
-
Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation.Acta Biomater. 2019 Jun;91:99-111. doi: 10.1016/j.actbio.2019.04.048. Epub 2019 Apr 24. Acta Biomater. 2019. PMID: 31028907
-
Improving the corrosion behavior of magnesium alloys with a focus on AZ91 Mg alloy intended for biomedical application by microstructure modification and coating.Proc Inst Mech Eng H. 2022 Aug;236(8):1188-1208. doi: 10.1177/09544119221105705. Epub 2022 Jun 23. Proc Inst Mech Eng H. 2022. PMID: 35735119 Review.
-
In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications.J Biomed Mater Res A. 2022 Feb;110(2):462-487. doi: 10.1002/jbm.a.37297. Epub 2021 Aug 21. J Biomed Mater Res A. 2022. PMID: 34418295 Review.
Cited by
-
Beyond hype: unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids.J Nanobiotechnology. 2024 Aug 21;22(1):500. doi: 10.1186/s12951-024-02759-z. J Nanobiotechnology. 2024. PMID: 39169401 Free PMC article. Review.
-
Finite element modeling of stress distribution and safety factors in a Ti-27Nb alloy hip implant under real-world physiological loading scenarios.PLoS One. 2024 Aug 6;19(8):e0300270. doi: 10.1371/journal.pone.0300270. eCollection 2024. PLoS One. 2024. PMID: 39106270 Free PMC article.
References
-
- Wu A.-M., Bisignano C., James S.L., Abady G.G., Abedi A., Abu-Gharbieh E., Alhassan R.K., Alipour V., Arabloo J., Asaad M., et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2:e580–e592. doi: 10.1016/S2666-7568(21)00172-0. - DOI - PMC - PubMed
-
- Lightsey H.M., Yeung C.M., von Keudell A. A Brief Guide to Initial Management of Orthopedic Injuries in the Emergency Department. Orthop. J. Harv. Med. Sch. 2019;20:52–61.
LinkOut - more resources
Full Text Sources
Miscellaneous

