Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood-Brain Barrier Model
- PMID: 37508911
- PMCID: PMC10377091
- DOI: 10.3390/brainsci13070980
Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood-Brain Barrier Model
Abstract
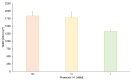
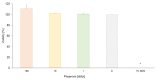
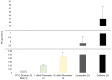
Phoenixin-14 is a recently discovered peptide regulating appetite. Interestingly, it is expressed in the gastrointestinal tract; however, its supposed receptor, GPR173, is predominantly found in hypothalamic areas. To date, it is unknown how peripherally secreted phoenixin-14 is able to reach its centrally located receptor. To investigate whether phoenixin is able to pass the blood-brain barrier, we used an in vitro mono-culture blood-brain barrier (BBB) model consisting of brain capillary-like endothelial cells derived from human induced-pluripotent stem cells (hiPSC-BCECs). The passage of 1 nMol and 10 nMol of phoenixin-14 via the mono-culture was measured after 30, 60, 90, 120, 150, 180, 210, and 240 min using a commercial ELISA kit. The permeability coefficients (PC) of 1 nMol and 10 nMol phoenixin-14 were 0.021 ± 0.003 and 0.044 ± 0.013 µm/min, respectively. In comparison with the PC of solutes known to cross the BBB in vivo, those of phoenixin-14 in both concentrations are very low. Here, we show that phoenixin-14 alone is not able to cross the BBB, suggesting that the effects of peripherally secreted phoenixin-14 depend on a co-transport mechanism at the BBB in vivo. The mechanisms responsible for phoenixin-14's orexigenic property along the gut-brain axis warrant further research.
Keywords: appetite regulation; blood–brain barrier; brain–gut axis; gastrointestinal tract; hypothalamus; in vitro techniques; induced pluripotent stem cells; peptides; phoenixin-14.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis.Clin Exp Pharmacol Physiol. 2019 Apr;46(4):350-359. doi: 10.1111/1440-1681.13061. Epub 2019 Feb 6. Clin Exp Pharmacol Physiol. 2019. PMID: 30609107
-
Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability.Arch Biochem Biophys. 2020 Mar 30;682:108275. doi: 10.1016/j.abb.2020.108275. Epub 2020 Jan 18. Arch Biochem Biophys. 2020. PMID: 31962109
-
Development of Human in vitro Brain-blood Barrier Model from Induced Pluripotent Stem Cell-derived Endothelial Cells to Predict the in vivo Permeability of Drugs.Neurosci Bull. 2019 Dec;35(6):996-1010. doi: 10.1007/s12264-019-00384-7. Epub 2019 May 11. Neurosci Bull. 2019. PMID: 31079318 Free PMC article.
-
Phoenixin-A Pleiotropic Gut-Brain Peptide.Int J Mol Sci. 2018 Jun 11;19(6):1726. doi: 10.3390/ijms19061726. Int J Mol Sci. 2018. PMID: 29891773 Free PMC article. Review.
-
Phoenixin: A Newly Discovered Peptide with Multi-Functions.Protein Pept Lett. 2017;24(6):472-475. doi: 10.2174/0929866524666170207154417. Protein Pept Lett. 2017. PMID: 28176660 Review.
Cited by
-
Immunolocalization and quantification of the phoenixin and GPR173 in the gastrointestinal tract of Holstein-Friesian bulls.BMC Vet Res. 2025 Feb 18;21(1):76. doi: 10.1186/s12917-025-04545-x. BMC Vet Res. 2025. PMID: 39966825 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

