Altered TRPM7-Dependent Calcium Influx in Natural Killer Cells of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients
- PMID: 37509075
- PMCID: PMC10377690
- DOI: 10.3390/biom13071039
Altered TRPM7-Dependent Calcium Influx in Natural Killer Cells of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients
Abstract
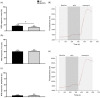
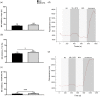
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disabling multisystemic condition. The pathomechanism of ME/CFS remains unestablished; however, impaired natural killer (NK) cell cytotoxicity is a consistent feature of this condition. Calcium (Ca2+) is crucial for NK cell effector functions. Growing research recognises Ca2+ signalling dysregulation in ME/CFS patients and implicates transient receptor potential ion channel dysfunction. TRPM7 (melastatin) was recently considered in the pathoaetiology of ME/CFS as it participates in several Ca2+-dependent processes that are central to NK cell cytotoxicity which may be compromised in ME/CFS. TRPM7-dependent Ca2+ influx was assessed in NK cells isolated from n = 9 ME/CFS patients and n = 9 age- and sex-matched healthy controls (HCs) using live cell fluorescent imaging techniques. Slope (p < 0.05) was significantly reduced in ME/CFS patients compared with HCs following TRPM7 activation. Half-time of maximal response (p < 0.05) and amplitude (p < 0.001) were significantly reduced in the HCs compared with the ME/CFS patients following TRPM7 desensitisation. Findings from this investigation suggest that TRPM7-dependent Ca2+ influx is reduced with agonism and increased with antagonism in ME/CFS patients relative to the age- and sex-matched HCs. The outcomes reported here potentially reflect TRPM3 dysfunction identified in this condition suggesting that ME/CFS is a TRP ion channelopathy.
Keywords: calcium; chronic fatigue syndrome; myalgic encephalomyelitis; natural killer cell; transient receptor potential melastatin 7.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial of financial relationships that could be construed as potential conflict of interest.
Figures
References
-
- Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., Staines D., Powles A.P., Speight N., Vallings R., et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. - DOI - PMC - PubMed
-
- Abdulla J., Torpy B.D. Chronic Fatigue Syndrome. In: De Groot L.J., Chrousos G., Dungan K., Feingold K.R., Grossman A., Hershman J.M., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000.
-
- Monro J.A., Puri B.K. A Molecular Neurobiological Approach to Understanding the Aetiology of Chronic Fatigue Syndrome (Myalgic Encephalomyelitis or Systemic Exertion Intolerance Disease) with Treatment Implications. Mol. Neurobiol. 2018;55:7377–7388. doi: 10.1007/s12035-018-0928-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

