Further Characterization of Fungal Halogenase RadH and Its Homologs
- PMID: 37509117
- PMCID: PMC10377541
- DOI: 10.3390/biom13071081
Further Characterization of Fungal Halogenase RadH and Its Homologs
Abstract
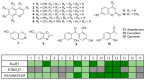
RadH is one of the flavin-dependent halogenases that has previously exhibited promising catalytic activity towards hydroxycoumarin, hydroxyisoquinoline, and phenolic derivatives. Here, we evaluated new functional homologs of RadH and expanded its specificities for the halogenation of non-tryptophan-derived, heterocyclic scaffolds. Our investigation revealed that RadH could effectively halogenate hydroxyquinoline and hydroxybenzothiophene. Assay optimization studies revealed the need to balance the various co-factor concentrations and where a GDHi co-factor recycling system most significantly improves the conversion and efficiency of the reaction. A crystal structure of RadH was also obtained with a resolution of 2.4 Å, and docking studies were conducted to pinpoint the binding and catalytic sites for substrates.
Keywords: X-ray crystallography; flavin-dependent halogenases; halogenation; heteroaromatic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jeschke P. Manufacturing Approaches of New Halogenated Agrochemicals. Eur. J. Org. Chem. 2022;2022:e202101513. doi: 10.1002/ejoc.202101513. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

