Exploration of Bis-Cinnamido-Polyamines as Intrinsic Antimicrobial Agents and Antibiotic Enhancers
- PMID: 37509123
- PMCID: PMC10377643
- DOI: 10.3390/biom13071087
Exploration of Bis-Cinnamido-Polyamines as Intrinsic Antimicrobial Agents and Antibiotic Enhancers
Abstract
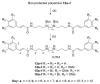
The marine natural product ianthelliformisamine C is a bis-cinnamido substituted spermine derivative that exhibits intrinsic antimicrobial properties and can enhance the action of doxycycline towards the Gram-negative bacterium Pseudomonas aeruginosa. As part of a study to explore the structure-activity requirements of these activities, we have synthesized a set of analogues that vary in the presence/absence of methoxyl group and bromine atoms and in the polyamine chain length. Intrinsic antimicrobial activity towards Staphylococcus aureus, methicillin-resistant S. aureus (MRSA) and the fungus Cryptococcus neoformans was observed for only the longest polyamine chain examples of non-brominated analogues while all examples bearing either one or two bromine atoms were active. Weak to no activity was typically observed towards Gram-negative bacteria, with exceptions being the longest polyamine chain examples 13f, 14f and 16f against Escherichia coli (MIC 1.56, 7.2 and 5.3 µM, respectively). Many of these longer polyamine-chain analogues also exhibited cytotoxic and/or red blood cell hemolytic properties, diminishing their potential as antimicrobial lead compounds. Two of the non-toxic, non-halogenated analogues, 13b and 13d, exhibited a strong ability to enhance the action of doxycycline against P. aeruginosa, with >64-fold and >32-fold enhancement, respectively. These results suggest that any future efforts to optimize the antibiotic-enhancing properties of cinnamido-polyamines should explore a wider range of aromatic ring substituents that do not include bromine or methoxyl groups.
Keywords: antibiotics; antifungal agents; antimicrobial; indole; polyamine; potentiator; structure-activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

