Microbiota and IL-33/31 Axis Linkage: Implications and Therapeutic Perspectives in Atopic Dermatitis and Psoriasis
- PMID: 37509136
- PMCID: PMC10377073
- DOI: 10.3390/biom13071100
Microbiota and IL-33/31 Axis Linkage: Implications and Therapeutic Perspectives in Atopic Dermatitis and Psoriasis
Abstract
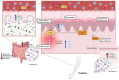
Microbiome dysbiosis and cytokine alternations are key features of atopic dermatitis (AD) and psoriasis (PsO), two of the most prevalent and burdensome pruritic skin conditions worldwide. Interleukin (IL)-33 and IL-31 have been recognized to be major players who act synergistically in the pathogenesis and maintenance of different chronic inflammatory conditions and pruritic skin disorders, including AD and PsO, and their potential role as therapeutic targets is being thoroughly investigated. The bidirectional interplay between dysbiosis and immunological changes has been extensively studied, but there is still debate regarding which of these two factors is the actual causative culprit behind the aetiopathological process that ultimately leads to AD and PsO. We conducted a literature review on the Pubmed database assessing articles of immunology, dermatology, microbiology and allergology with the aim to strengthen the hypothesis that dysbiosis is at the origin of the IL-33/IL-31 dysregulation that contributes to the pathogenesis of AD and PsO. Finally, we discussed the therapeutic options currently in development for the treatment of these skin conditions targeting IL-31, IL-33 and/or the microbiome.
Keywords: IL-31; IL-33; atopic dermatitis; cytokines; inflammation; microbiota; pathogenesis; psoriasis; skin; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Eichenfield L.F., Tom W.L., Chamlin S.L., Feldman S.R., Hanifin J.M., Simpson E.L., Berger T.G., Bergman J.N., Cohen D.E., Cooper K.D., et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014;70:338–351. doi: 10.1016/j.jaad.2013.10.010. - DOI - PMC - PubMed
-
- Stuart B.L., Howells L., Pattinson R.L., Chalmers J.R., Grindlay D., Rogers N.K., Grinich E., Pawlitschek T., Simpson E.L., Thomas K.S. Measurement properties of patient-reported outcome measures for eczema control: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021;35:1987–1993. doi: 10.1111/jdv.17335. - DOI - PubMed
-
- Li A., Zhang M., Yang Y., Zhang J., Xie X., Li X., Zhang H. Patient-reported outcome (PRO) instruments for disease severity and quality of life in patients with atopic dermatitis: A systematic review of English and Chinese literature. Ann. Transl. Med. 2022;10:906. doi: 10.21037/atm-22-3164. - DOI - PMC - PubMed
-
- Williams H.C., Schmitt J., Thomas K.S., Spuls P.I., Simpson E.L., Apfelbacher C.J., Chalmers J.R., Furue M., Katoh N., Gerbens L.A.A., et al. The HOME Core outcome set for clinical trials of atopic dermatitis. J. Allergy Clin. Immunol. 2022;149:1899–1911. doi: 10.1016/j.jaci.2022.03.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

