A Frame-by-Frame Glance at Membrane Fusion Mechanisms: From Viral Infections to Fertilization
- PMID: 37509166
- PMCID: PMC10377500
- DOI: 10.3390/biom13071130
A Frame-by-Frame Glance at Membrane Fusion Mechanisms: From Viral Infections to Fertilization
Abstract
Viral entry and fertilization are distinct biological processes that share a common mechanism: membrane fusion. In viral entry, enveloped viruses attach to the host cell membrane, triggering a series of conformational changes in the viral fusion proteins. This results in the exposure of a hydrophobic fusion peptide, which inserts into the host membrane and brings the viral and host membranes into close proximity. Subsequent structural rearrangements in opposing membranes lead to their fusion. Similarly, membrane fusion occurs when gametes merge during the fertilization process, though the exact mechanism remains unclear. Structural biology has played a pivotal role in elucidating the molecular mechanisms underlying membrane fusion. High-resolution structures of the viral and fertilization fusion-related proteins have provided valuable insights into the conformational changes that occur during this process. Understanding these mechanisms at a molecular level is essential for the development of antiviral therapeutics and tools to influence fertility. In this review, we will highlight the biological importance of membrane fusion and how protein structures have helped visualize both common elements and subtle divergences in the mechanisms behind fusion; in addition, we will examine the new tools that recent advances in structural biology provide researchers interested in a frame-by-frame understanding of membrane fusion.
Keywords: cryo-electron microscopy; fertilization; fusion mechanism; membrane fusion; structural biology; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
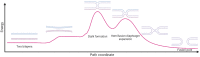
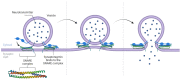

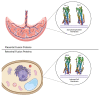

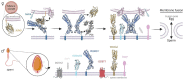
References
-
- Plemper R.K. Membrane Organization|Membrane Fusion. In: Jez J., editor. Encyclopedia of Biological Chemistry III. 3rd ed. Elsevier; Oxford, UK: 2021. pp. 804–812.

