The Promise of Retinoids in the Treatment of Cancer: Neither Burnt Out Nor Fading Away
- PMID: 37509198
- PMCID: PMC10377082
- DOI: 10.3390/cancers15143535
The Promise of Retinoids in the Treatment of Cancer: Neither Burnt Out Nor Fading Away
Abstract
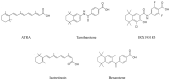
Since the introduction of all-trans retinoic acid (ATRA), acute promyelocytic leukemia (APL) has become a highly curable malignancy, especially in combination with arsenic trioxide (ATO). ATRA's success has deepened our understanding of the role of the RARα pathway in normal hematopoiesis and leukemogenesis, and it has influenced a generation of cancer drug development. Retinoids have also demonstrated some efficacy in a handful of other disease entities, including as a maintenance therapy for neuroblastoma and in the treatment of cutaneous T-cell lymphomas; nevertheless, the promise of retinoids as a differentiating therapy in acute myeloid leukemia (AML) more broadly, and as a cancer preventative, have largely gone unfulfilled. Recent research into the mechanisms of ATRA resistance and the biomarkers of RARα pathway dysregulation in AML have reinvigorated efforts to successfully deploy retinoid therapy in a broader subset of myeloid malignancies. Recent studies have demonstrated that the bone marrow environment is highly protected from exogenous ATRA via local homeostasis controlled by stromal cells expressing CYP26, a key enzyme responsible for ATRA inactivation. Synthetic CYP26-resistant retinoids such as tamibarotene bypass this stromal protection and have shown superior anti-leukemic effects. Furthermore, recent super-enhancer (SE) analysis has identified a novel AML subgroup characterized by high expression of RARα through strong SE levels in the gene locus and increased sensitivity to tamibarotene. Combined with a hypomethylating agent, synthetic retinoids have shown synergistic anti-leukemic effects in non-APL AML preclinical models and are now being studied in phase II and III clinical trials.
Keywords: APL; ATRA; CYP26; RARα; differentiation therapy; non-APL AML; retinoid; tamibarotene.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- Burnett A.K., Hills R.K., Green C., Jenkinson S., Koo K., Patel Y., Guy C., Gilkes A., Milligan D.W., Goldstone A.H., et al. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: Overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood. 2010;115:948–956. - PubMed
-
- Burnett A.K., Hills R.K., Milligan D.W., Goldstone A.H., Prentice A.G., McMullin M.F., Duncombe A., Gibson B., Wheatley K. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: Results of the MRC AML12 trial. J. Clin. Oncol. 2010;28:586–595. - PubMed
-
- Fukasawa H., Iijima T., Kagechika H., Hashimoto Y., Shudo K. Expression of the ligand-binding domain-containing region of retinoic acid receptors alpha, beta and gamma in Escherichia coli and evaluation of ligand-binding selectivity. Biol. Pharm. Bull. 1993;16:343–348. - PubMed
-
- Chee L.C.Y., Hendy J., Purton L.E., McArthur G.A. ATRA and the specific RARα agonist, NRX195183, have opposing effects on the clonogenicity of pre-leukemic murine AML1-ETO bone marrow cells. Leukemia. 2013;27:1369–1380. - PubMed
Publication types
LinkOut - more resources
Full Text Sources