The Landscape of Small Leucine-Rich Proteoglycan Impact on Cancer Pathogenesis with a Focus on Biglycan and Lumican
- PMID: 37509212
- PMCID: PMC10377491
- DOI: 10.3390/cancers15143549
The Landscape of Small Leucine-Rich Proteoglycan Impact on Cancer Pathogenesis with a Focus on Biglycan and Lumican
Abstract
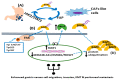
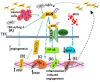
Cancer development is a multifactorial procedure that involves changes in the cell microenvironment and specific modulations in cell functions. A tumor microenvironment contains tumor cells, non-malignant cells, blood vessels, cells of the immune system, stromal cells, and the extracellular matrix (ECM). The small leucine-rich proteoglycans (SLRPs) are a family of nineteen proteoglycans, which are ubiquitously expressed among mammalian tissues and especially abundant in the ECM. SLRPs are divided into five canonical classes (classes I-III, containing fourteen members) and non-canonical classes (classes IV-V, including five members) based on their amino-acid structural sequence, chromosomal organization, and functional properties. Variations in both the protein core structure and glycosylation status lead to SLRP-specific interactions with cell membrane receptors, cytokines, growth factors, and structural ECM molecules. SLRPs have been implicated in the regulation of cancer growth, motility, and invasion, as well as in cancer-associated inflammation and autophagy, highlighting their crucial role in the processes of carcinogenesis. Except for the class I SLRP decorin, to which an anti-tumorigenic role has been attributed, other SLPRs' roles have not been fully clarified. This review will focus on the functions of the class I and II SLRP members biglycan and lumican, which are correlated to various aspects of cancer development.
Keywords: biglycan; cancer; cancer invasion; cancer-associated inflammation; extracelllar matrix; lumican; small-leucine rich proteoglycans (SLRPs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Small Leucine Rich Proteoglycans (decorin, biglycan and lumican) in cancer.Clin Chim Acta. 2019 Apr;491:1-7. doi: 10.1016/j.cca.2019.01.003. Epub 2019 Jan 7. Clin Chim Acta. 2019. PMID: 30629950 Review.
-
Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site.Arthritis Res Ther. 2006;8(1):R26. doi: 10.1186/ar1873. Epub 2006 Jan 3. Arthritis Res Ther. 2006. PMID: 16507124 Free PMC article.
-
Molecular cues for immune cells from small leucine-rich repeat proteoglycans in their extracellular matrix-associated and free forms.Matrix Biol. 2023 Nov;123:48-58. doi: 10.1016/j.matbio.2023.10.001. Epub 2023 Oct 2. Matrix Biol. 2023. PMID: 37793508 Free PMC article. Review.
-
Colocalization of the collagen-binding proteoglycans decorin, biglycan, fibromodulin and lumican with different cells in human gingiva.J Periodontal Res. 2005 Feb;40(1):73-86. doi: 10.1111/j.1600-0765.2004.00776.x. J Periodontal Res. 2005. PMID: 15613083
-
Small leucine-rich proteoglycans (SLRPs) in uterine tissues during pregnancy in mice.Reproduction. 2003 Apr;125(4):585-95. doi: 10.1530/rep.0.1250585. Reproduction. 2003. PMID: 12683929
Cited by
-
PRELP regulated by GAS5/miR-3127-5p suppresses cisplatin resistance in oral squamous cell carcinoma.Cytotechnology. 2025 Jun;77(3):92. doi: 10.1007/s10616-025-00749-z. Epub 2025 Apr 28. Cytotechnology. 2025. PMID: 40309012
-
Decorin impeded the advancement of thyroid papillary carcinoma by thwarting the EGFR/ FER/ SHP2 signaling-induced sustenance of early endosomes.Heliyon. 2024 Jun 20;10(13):e33358. doi: 10.1016/j.heliyon.2024.e33358. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39035505 Free PMC article.
-
Targeting extracellular matrix stiffness for cancer therapy.Front Immunol. 2024 Dec 2;15:1467602. doi: 10.3389/fimmu.2024.1467602. eCollection 2024. Front Immunol. 2024. PMID: 39697341 Free PMC article. Review.
-
Biglycan promotes tumour proliferation and invasion in human colon cancers.J Int Med Res. 2024 Nov;52(11):3000605241300067. doi: 10.1177/03000605241300067. J Int Med Res. 2024. PMID: 39612315 Free PMC article.
-
Secreted lumican from the tumor microenvironment potentiates HCC stemness and progression.Hepatol Commun. 2025 Aug 15;9(9):e0778. doi: 10.1097/HC9.0000000000000778. eCollection 2025 Sep 1. Hepatol Commun. 2025. PMID: 40824257 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

