The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer-A Narrative Review
- PMID: 37509224
- PMCID: PMC10377299
- DOI: 10.3390/cancers15143561
The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer-A Narrative Review
Abstract
Non-small cell lung cancer (NSCLC) is a leading cause of death, but over the past decade, there has been tremendous progress in the field with new targeted therapies. The mesenchymal-epithelial transition factor (MET) proto-oncogene has been implicated in multiple solid tumors, including NSCLC, and dysregulation in NSCLC from MET can present most notably as MET exon 14 skipping mutation and amplification. From this, MET tyrosine kinase inhibitors (TKIs) have been developed to treat this dysregulation despite challenges with efficacy and reliable biomarkers. Capmatinib is a Type Ib MET TKI first discovered in 2011 and was FDA approved in August 2022 for advanced NSCLC with MET exon 14 skipping mutation. In this narrative review, we discuss preclinical and early-phase studies that led to the GEOMETRY mono-1 study, which showed beneficial efficacy in MET exon 14 skipping mutations, leading to FDA approval of capmatinib along with Foundation One CDx assay as its companion diagnostic assay. Current and future directions of capmatinib are focused on improving the efficacy, overcoming the resistance of capmatinib, and finding approaches for new indications of capmatinib such as acquired MET amplification from epidermal growth factor receptor (EGFR) TKI resistance. Clinical trials now involve combination therapy with capmatinib, including amivantamab, trametinib, and immunotherapy. Furthermore, new drug agents, particularly antibody-drug conjugates, are being developed to help treat patients with acquired resistance from capmatinib and other TKIs.
Keywords: MET dysregulation; NSCLC; capmatinib; detection; tyrosine kinase inhibitor.
Conflict of interest statement
RH is a consultant for Targeted Oncology and received honoraria from DAVA Oncology and The Dedham Group. D.J.B. has received consulting fees from Seagen. MN has received consulting fees from AstraZeneca, Caris Life Sciences, Daiichi Sankyo, Novartis, EMD Serono, Pfizer, Lilly and Genentech, has received travel support from AnHeart Therapeutics and is a speaker for Takeda, Janssen, Mirati and Blueprint Medicines.
Figures
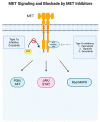
References
-
- Planchard D., Smit E.F., Groen H.J.M., Mazieres J., Besse B., Helland Å., Giannone V., D’Amelio A.M., Zhang P., Mookerjee B., et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. - DOI - PubMed
-
- Shaw A.T., Riely G.J., Bang Y.-J., Kim D.-W., Camidge D.R., Solomon B.J., Varella-Garcia M., Iafrate A.J., Shapiro G.I., Usari T., et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. - DOI - PMC - PubMed
-
- Drilon A., Siena S., Dziadziuszko R., Barlesi F., Krebs M.G., Shaw A.T., de Braud F., Rolfo C., Ahn M.-J., Wolf J., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

