Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma
- PMID: 37509257
- PMCID: PMC10377190
- DOI: 10.3390/cancers15143594
Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma
Abstract
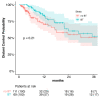
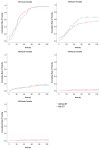
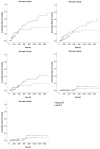
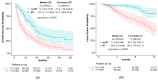
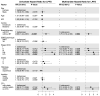
This study aims to investigate the effect of dose escalation with brachytherapy (BT) as an addition to definitive chemoradiotherapy (CRT) on local control and survival in esophageal cancer. From 2001 to 2020, 183 patients with locally limited or locally advanced esophageal cancer received definitive CRT with or without brachytherapy in a two-center study. External-beam radiotherapy was delivered at 50.4 Gy in 1.8 Gy daily fractions, followed by a sequential boost to the primary tumor of 9 Gy in 1.8 Gy daily fractions if indicated. Intraluminal high dose rate (HDR) Ir-192 brachytherapy was performed on 71 patients at 10 Gy in two fractions, with one fraction per week. The combined systemic therapy schedules used included 5-fluorouracil/cisplatin or 5-fluorouracil alone. Cisplatin was not administered in patients receiving brachytherapy. The median local progression-free survival was significantly extended in the BT group (18.7 vs. 6.0 months; p < 0.0001), and the median local control was also significantly prolonged (30.5 vs. 11.3 months, p = 0.008). Overall survival (OS) significantly increased in the BT group (median OS 22.7 vs. 9.1 months, p < 0.0001). No significant difference in the overall rate of acute toxicities was observed; however, the rate of acute esophagitis was significantly higher in the BT group (94.4% vs. 81.2%). Likewise, the overall rate of late toxicities (43.7% vs. 18.8%) was significantly higher in the BT group, including the rate of esophageal stenosis (22.5% vs. 9.8%). There was no difference in the occurrence of life-threatening or lethal late toxicities (grades 4 and 5). Brachytherapy, after chemoradiation with single-agent 5-FU, represents a safe and effective alternative for dose escalation in the definitive treatment of esophageal cancer.
Keywords: brachytherapy; chemoradiotherapy; dose escalation; esophageal cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cooper J.S., Guo M.D., Herskovic A., Macdonald J.S., Martenson J.J.A., Jr., Al-Sarraf M., Byhardt R., Russell A.H., Beitler J.J., Spencer S., et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-term follow-up of a prospective randomized trial (Rtog 85-01) JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. - DOI - PubMed
-
- Minsky B.D., Pajak T.F., Ginsberg R.J., Pisansky T.M., Martenson J., Komaki R., Okawara G., Rosenthal S.A., Kelsen D.P. Int 0123 (Radiation Therapy Oncology Group 94-05) Phase Iii Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. - DOI - PubMed
-
- Versteijne E., van Laarhoven H.W.M., van Hooft J.E., van Os R.M., Geijsen E.D., Henegouwen M.I.v.B., Hulshof M.C.C.M. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: Locoregional recurrence pattern. Dis. Esophagus. 2015;28:453–459. doi: 10.1111/dote.12215. - DOI - PubMed
-
- Teoh A.Y.B., Chiu P.W.Y., Yeung W.K., Liu S.Y.W., Wong S.K.H., Ng E.K.W. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: Results from a randomized controlled trial. Ann. Oncol. 2013;24:165–171. doi: 10.1093/annonc/mds206. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

