Decreased Gene Expression of Antiangiogenic Factors in Endometrial Cancer: qPCR Analysis and Machine Learning Modelling
- PMID: 37509322
- PMCID: PMC10378066
- DOI: 10.3390/cancers15143661
Decreased Gene Expression of Antiangiogenic Factors in Endometrial Cancer: qPCR Analysis and Machine Learning Modelling
Abstract
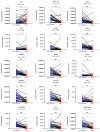
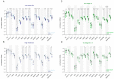
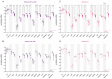
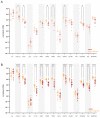
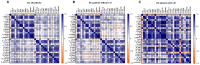
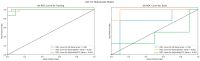
Endometrial cancer (EC) is an increasing health concern, with its growth driven by an angiogenic switch that occurs early in cancer development. Our study used publicly available datasets to examine the expression of angiogenesis-related genes and proteins in EC tissues, and compared them with adjacent control tissues. We identified nine genes with significant differential expression and selected six additional antiangiogenic genes from prior research for validation on EC tissue in a cohort of 36 EC patients. Using machine learning, we built a prognostic model for EC, combining our data with The Cancer Genome Atlas (TCGA). Our results revealed a significant up-regulation of IL8 and LEP and down-regulation of eleven other genes in EC tissues. These genes showed differential expression in the early stages and lower grades of EC, and in patients without deep myometrial or lymphovascular invasion. Gene co-expressions were stronger in EC tissues, particularly those with lymphovascular invasion. We also found more extensive angiogenesis-related gene involvement in postmenopausal women. In conclusion, our findings suggest that angiogenesis in EC is predominantly driven by decreased antiangiogenic factor expression, particularly in EC with less favourable prognostic features. Our machine learning model effectively stratified EC based on gene expression, distinguishing between low and high-grade cases.
Keywords: LEP; TCGA; angiogenic factor; endometrial cancer; machine learning; tumour-adjacent tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Models including preoperative plasma levels of angiogenic factors, leptin and IL-8 as potential biomarkers of endometrial cancer.Front Oncol. 2022 Nov 24;12:972131. doi: 10.3389/fonc.2022.972131. eCollection 2022. Front Oncol. 2022. PMID: 36505829 Free PMC article.
-
The impact of uterine adenomyosis on the histopathological risk factors and survival in patients with endometrial adenocarcinoma.J Obstet Gynaecol. 2022 Aug;42(6):2213-2219. doi: 10.1080/01443615.2022.2036959. Epub 2022 Mar 9. J Obstet Gynaecol. 2022. PMID: 35260040
-
Integrated multi-omics analyses and functional validation reveal TTK as a novel EMT activator for endometrial cancer.J Transl Med. 2023 Feb 25;21(1):151. doi: 10.1186/s12967-023-03998-8. J Transl Med. 2023. PMID: 36829176 Free PMC article.
-
Prognostic value of myometrial invasion and TCGA groups of endometrial carcinoma.Gynecol Oncol. 2021 Aug;162(2):401-406. doi: 10.1016/j.ygyno.2021.05.029. Epub 2021 Jun 1. Gynecol Oncol. 2021. PMID: 34088515
-
Histological Prognostic Factors of Endometrial Cancer in Patients with Adenomyosis: A Systematic Review and Meta-Analysis.Pathobiology. 2022;89(3):127-134. doi: 10.1159/000521105. Epub 2022 Jan 20. Pathobiology. 2022. PMID: 35051948
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

