Subcutaneous Tocilizumab May Be Effective in Refractory Fibromyalgia Patients
- PMID: 37509414
- PMCID: PMC10376766
- DOI: 10.3390/biomedicines11071774
Subcutaneous Tocilizumab May Be Effective in Refractory Fibromyalgia Patients
Abstract
Introduction: Fibromyalgia (FM) is a chronic disorder characterized by widespread pain with an enormous symptom burden. Its treatment efficacy is limited. Its pathogenesis involves immune dysregulation, which includes interleukin-6 (IL-6) production.
Methods: We herein reported a case series of FM patients receiving subcutaneous tocilizumab at our institution. FM symptoms were evaluated by the revised Fibromyalgia Impact Questionnaire (FIQR), which included pain level, and the fibromyalgianess scale based on the 2016 criteria of the American College of Rheumatology (ACR). FM symptoms were compared using the Wilcoxon signed-rank test. Neutrophils from primary FM patients and matched healthy controls were also isolated for transcriptome analysis.
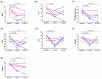
Results: We presented a total of two primary and four secondary FM patients who had received subcutaneous tocilizumab for a minimum of 12 weeks. All patients had severe symptoms despite standard treatments. Patients' FIQR and fibromyalgianess both dropped at 4 and 12 weeks. Four (67%) of them reached a pain reduction of ≥30% at 4 weeks, and three (50%) reached a pain reduction of ≥30% at 12 weeks. Possible differentially expressed genes were identified in primary FM patients when compared with controls and after tocilizumab treatment.
Conclusions: FM patients likely benefited from subcutaneous tocilizumab therapy. A randomized controlled trial is needed to verify its efficacy.
Keywords: fibromyalgia; neutrophils; pain; tocilizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Direct-Acting Antiviral Drugs Reduce Fibromyalgia Symptoms in Patients with Chronic Hepatitis C.J Clin Med. 2022 Sep 10;11(18):5327. doi: 10.3390/jcm11185327. J Clin Med. 2022. PMID: 36142974 Free PMC article.
-
A questionnaire using the modified 2010 American College of Rheumatology criteria for fibromyalgia: specificity and sensitivity in clinical practice.J Rheumatol. 2013 Sep;40(9):1590-5. doi: 10.3899/jrheum.130367. Epub 2013 Jul 1. J Rheumatol. 2013. PMID: 23818707
-
A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients.Scand J Pain. 2016 Oct;13:166-172. doi: 10.1016/j.sjpain.2016.07.004. Epub 2016 Aug 22. Scand J Pain. 2016. PMID: 28850525
-
Disentangling Diagnosis and Management of Fibromyalgia.J Rheum Dis. 2022 Jan 1;29(1):4-13. doi: 10.4078/jrd.2022.29.1.4. J Rheum Dis. 2022. PMID: 37476701 Free PMC article. Review.
-
Effect of coenzyme Q10 evaluated by 1990 and 2010 ACR Diagnostic Criteria for Fibromyalgia and SCL-90-R: four case reports and literature review.Nutrition. 2013 Nov-Dec;29(11-12):1422-5. doi: 10.1016/j.nut.2013.05.005. Nutrition. 2013. PMID: 24103521 Review.
Cited by
-
Assessing the Impact of IL-6 and Serotonin on Pain and Symptomatology in Fibromyalgia: An Exploratory Clinical Study.J Pers Med. 2024 Aug 22;14(8):886. doi: 10.3390/jpm14080886. J Pers Med. 2024. PMID: 39202077 Free PMC article.
-
Inflammation, Autoimmunity, and Infection in Fibromyalgia: A Narrative Review.Int J Mol Sci. 2024 May 29;25(11):5922. doi: 10.3390/ijms25115922. Int J Mol Sci. 2024. PMID: 38892110 Free PMC article. Review.
-
How to Distinguish Non-Inflammatory from Inflammatory Pain in RA?Curr Rheumatol Rep. 2024 Dec;26(12):403-413. doi: 10.1007/s11926-024-01159-4. Epub 2024 Aug 9. Curr Rheumatol Rep. 2024. PMID: 39120749 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

