Hydrogen Sulfide Ameliorates SARS-CoV-2-Associated Lung Endothelial Barrier Disruption
- PMID: 37509430
- PMCID: PMC10376201
- DOI: 10.3390/biomedicines11071790
Hydrogen Sulfide Ameliorates SARS-CoV-2-Associated Lung Endothelial Barrier Disruption
Abstract
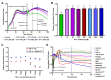
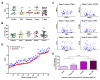
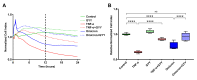
Recent studies have confirmed that lung microvascular endothelial injury plays a critical role in the pathophysiology of COVID-19. Our group and others have demonstrated the beneficial effects of H2S in several pathological processes and provided a rationale for considering the therapeutic implications of H2S in COVID-19 therapy. Here, we evaluated the effect of the slow-releasing H2S donor, GYY4137, on the barrier function of a lung endothelial cell monolayer in vitro, after challenging the cells with plasma samples from COVID-19 patients or inactivated SARS-CoV-2 virus. We also assessed how the cytokine/chemokine profile of patients' plasma, endothelial barrier permeability, and disease severity correlated with each other. Alterations in barrier permeability after treatments with patient plasma, inactivated virus, and GYY4137 were monitored and assessed by electrical impedance measurements in real time. We present evidence that GYY4137 treatment reduced endothelial barrier permeability after plasma challenge and completely reversed the endothelial barrier disruption caused by inactivated SARS-CoV-2 virus. We also showed that disease severity correlated with the cytokine/chemokine profile of the plasma but not with barrier permeability changes in our assay. Overall, these data demonstrate that treatment with H2S-releasing compounds has the potential to ameliorate SARS-CoV-2-associated lung endothelial barrier disruption.
Keywords: COVID-19; SARS-CoV-2; TNF-α; cytokine; endothelial barrier; hydrogen sulfide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization (WHO) Coronavirus (COVID-19) Dashboard. [(accessed on 18 May 2023)]. Available online: https://covid19.who.int.
-
- Center for Disease Control and Prevention (CDC), Long COVID or Post-COVID Conditions. [(accessed on 18 May 2023)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

