Exploring the Therapeutic Potential of Phosphorylated Cis-Tau Antibody in a Pig Model of Traumatic Brain Injury
- PMID: 37509447
- PMCID: PMC10376756
- DOI: 10.3390/biomedicines11071807
Exploring the Therapeutic Potential of Phosphorylated Cis-Tau Antibody in a Pig Model of Traumatic Brain Injury
Abstract
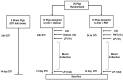
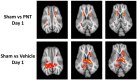
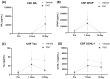
Traumatic brain injury (TBI) results in the generation of tau. As hyperphosphorylated tau (p-tau) is one of the major consequences of TBI, targeting p-tau in TBI may lead to the development of new therapy. Twenty-five pigs underwent a controlled cortical impact. One hour after TBI, pigs were administered either vehicle (n = 13) or PNT001 (n = 12), a monoclonal antibody for the cis conformer of tau phosphorylated at threonine 231. Plasma biomarkers of neural injury were assessed for 14 days. Diffusion tensor imaging was performed at day 1 and 14 after injury, and these were compared to historical control animals (n = 4). The fractional anisotropy data showed significant white matter injury for groups at 1 day after injury in the corona radiata. At 14 days, the vehicle-treated pigs, but not the PNT001-treated animals, exhibited significant white matter injury compared to sham pigs in the ipsilateral corona radiata. The PNT001-treated pigs had significantly lower levels of plasma glial fibrillary acidic protein (GFAP) at day 2 and day 4. These findings demonstrate a subtle reduction in the areas of white matter injury and biomarkers of neurological injury after treatment with PNT001 following TBI. These findings support additional studies for PNT001 as well as the potential use of this agent in clinical trials in the near future.
Keywords: PNT001; TBI; phosphorylated tau; tau; traumatic brain injury.
Conflict of interest statement
Michael Ahlijanian is an employee of Pinteon Therapeutics and received financial compensation.
Figures
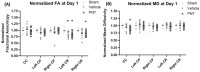



References
-
- Shin R.W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab. Investig. 1991;64:693–702. - PubMed
-
- Tagge C.A., Fisher A.M., Minaeva O.V., Gaudreau-Balderrama A., Moncaster J.A., Zhang X.L., Wojnarowicz M.W., Casey N., Lu H., Kokiko-Cochran O.N., et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141:422–458. doi: 10.1093/brain/awx350. - DOI - PMC - PubMed
-
- Mez J., Daneshvar D.H., Kiernan P.T., Abdolmohammadi B., Alvarez V.E., Huber B.R., Alosco M.L., Solomon T.M., Nowinski C.J., McHale L., et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017;318:360–370. doi: 10.1001/jama.2017.8334. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

