Relationship between Macrophages and Tissue Microenvironments in Diabetic Kidneys
- PMID: 37509528
- PMCID: PMC10377233
- DOI: 10.3390/biomedicines11071889
Relationship between Macrophages and Tissue Microenvironments in Diabetic Kidneys
Abstract
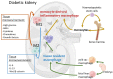
Diabetic nephropathy (DN) is the leading cause of end-stage kidney disease. Increasing evidence has suggested that inflammation is a key microenvironment involved in the development and progression of DN. Studies have confirmed that macrophage accumulation is closely related to the progression to human DN. Macrophage phenotype is highly regulated by the surrounding microenvironment in the diabetic kidneys. M1 and M2 macrophages represent distinct and sometimes coexisting functional phenotypes of the same population, with their roles implicated in pathological changes, such as in inflammation and fibrosis associated with the stage of DN. Recent findings from single-cell RNA sequencing of macrophages in DN further confirmed the heterogeneity and plasticity of the macrophages. In addition, intrinsic renal cells interact with macrophages directly or through changes in the tissue microenvironment. Macrophage depletion, modification of its polarization, and autophagy could be potential new therapies for DN.
Keywords: cell–cell interaction; diabetic nephropathy; fibrosis; inflammation; macrophages; microenvironment; single-cell RNA sequencing; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Coca S.G., Nadkarni G.N., Huang Y., Moledina D.G., Rao V., Zhang J., Ferket B., Crowley S.T., Fried L.F., Parikh C.R. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2017;28:2786–2793. doi: 10.1681/ASN.2016101101. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

