Fabrication of a Smart Fibrous Biomaterial That Harbors an Active TGF-β1 Peptide: A Promising Approach for Cartilage Regeneration
- PMID: 37509529
- PMCID: PMC10377373
- DOI: 10.3390/biomedicines11071890
Fabrication of a Smart Fibrous Biomaterial That Harbors an Active TGF-β1 Peptide: A Promising Approach for Cartilage Regeneration
Abstract
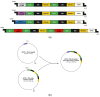
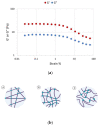
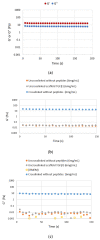
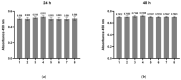
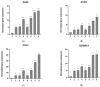
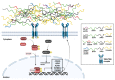
The regeneration of articular cartilage remains a serious problem in various pathological conditions such as osteoarthritis, due to the tissue's low self-healing capacity. The latest therapeutic approaches focus on the construction of biomaterials that induce cartilage repair. This research describes the design, synthesis, and investigation of a safe, "smart", fibrous scaffold containing a genetically incorporated active peptide for chondrogenic induction. While possessing specific sequences and the respective mechanical properties from natural fibrous proteins, the fibers also incorporate a Transforming Growth Factor-β1 (TGF-β1)-derived peptide (YYVGRKPK) that can promote chondrogenesis. The scaffold formed stable porous networks with shear-thinning properties at 37 °C, as shown by SEM imaging and rheological characterization, and were proven to be non-toxic to human dental pulp stem cells (hDPSCs). Its chondrogenic capacity was evidenced by a strong increase in the expression of specific chondrogenesis gene markers SOX9, COL2, ACAN, TGFBR1A, and TGFBR2 in cells cultured on "scaffold-TGFβ1" for 21 days and by increased phosphorylation of intracellular signaling proteins Smad-2 and Erk-1/2. Additionally, intense staining of glycosaminoglycans was observed in these cells. According to our results, "scaffold-TGFβ1" is proposed for clinical studies as a safe, injectable treatment for cartilage degeneration.
Keywords: TGF-β1 peptide; cartilage regeneration; chondrogenesis; elastin-like polypeptides; mussel-foot adhesive protein; silk fibroin; smart biomaterials; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




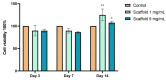







References
-
- Luna M. The Influence of the Gut Microbiome on Bone and Joint Disease. Cornell University; Ithaca, NY, USA: 2020.
-
- Gloria A., De Santis R., Ambrosio L. Polymer-Based Composite Scaffolds for Tissue Engineering. J. Appl. Biomater. Biomech. 2010;8:57–67. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

