Dysregulated Calcium Handling in Cirrhotic Cardiomyopathy
- PMID: 37509534
- PMCID: PMC10377313
- DOI: 10.3390/biomedicines11071895
Dysregulated Calcium Handling in Cirrhotic Cardiomyopathy
Abstract
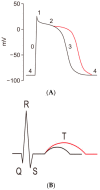
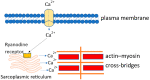
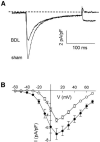
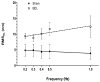
Cirrhotic cardiomyopathy is a syndrome of blunted cardiac systolic and diastolic function in patients with cirrhosis. However, the mechanisms remain incompletely known. Since contractility and relaxation depend on cardiomyocyte calcium transients, any factors that impact cardiac contractile and relaxation functions act eventually through calcium transients. In addition, calcium transients play an important role in cardiac arrhythmias. The present review summarizes the calcium handling system and its role in cardiac function in cirrhotic cardiomyopathy and its mechanisms. The calcium handling system includes calcium channels on the sarcolemmal plasma membrane of cardiomyocytes, the intracellular calcium-regulatory apparatus, and pertinent proteins in the cytosol. L-type calcium channels, the main calcium channel in the plasma membrane of cardiomyocytes, are decreased in the cirrhotic heart, and the calcium current is decreased during the action potential both at baseline and under stimulation of beta-adrenergic receptors, which reduces the signal to calcium-induced calcium release. The study of sarcomere length fluctuations and calcium transients demonstrated that calcium leakage exists in cirrhotic cardiomyocytes, which decreases the amount of calcium storage in the sarcoplasmic reticulum (SR). The decreased storage of calcium in the SR underlies the reduced calcium released from the SR, which results in decreased cardiac contractility. Based on studies of heart failure with non-cirrhotic cardiomyopathy, it is believed that the calcium leakage is due to the destabilization of interdomain interactions (dispersion) of ryanodine receptors (RyRs). A similar dispersion of RyRs may also play an important role in reduced contractility. Multiple defects in calcium handling thus contribute to the pathogenesis of cirrhotic cardiomyopathy.
Keywords: L-type calcium channel; calcium transient; cardiac contractility; cirrhotic cardiomyopathy; ryanodine receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Altered cellular calcium regulatory systems in a rat model of cirrhotic cardiomyopathy.Gastroenterology. 2001 Nov;121(5):1209-18. doi: 10.1053/gast.2001.28653. Gastroenterology. 2001. PMID: 11677214
-
The Cardiomyocyte in Cirrhosis: Pathogenic Mechanisms Underlying Cirrhotic Cardiomyopathy.Rev Cardiovasc Med. 2024 Dec 24;25(12):457. doi: 10.31083/j.rcm2512457. eCollection 2024 Dec. Rev Cardiovasc Med. 2024. PMID: 39742234 Free PMC article. Review.
-
Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells.Acta Pharmacol Sin. 2006 Jul;27(7):901-10. doi: 10.1111/j.1745-7254.2006.00380.x. Acta Pharmacol Sin. 2006. PMID: 16787575
-
Impaired myosin isoform shift and calcium transients contribute to cellular pathogenesis of rat cirrhotic cardiomyopathy.Liver Int. 2020 Nov;40(11):2808-2819. doi: 10.1111/liv.14599. Epub 2020 Aug 24. Liver Int. 2020. PMID: 32654385
-
Cardiac BIN1 (cBIN1) is a regulator of cardiac contractile function and an emerging biomarker of heart muscle health.Sci China Life Sci. 2017 Mar;60(3):257-263. doi: 10.1007/s11427-016-0249-x. Epub 2016 Nov 23. Sci China Life Sci. 2017. PMID: 27888388 Review.
Cited by
-
Evaluation of Electrocardiogram Parameters and Heart Rate Variability During Blood Pressure Elevation by Phenylephrine in Cirrhotic Rats.Cardiovasc Toxicol. 2024 Mar;24(3):321-334. doi: 10.1007/s12012-024-09839-4. Epub 2024 Feb 26. Cardiovasc Toxicol. 2024. PMID: 38409566
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

