New Onset Autoimmune Diseases after the Sputnik Vaccine
- PMID: 37509537
- PMCID: PMC10377489
- DOI: 10.3390/biomedicines11071898
New Onset Autoimmune Diseases after the Sputnik Vaccine
Abstract
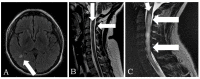
The vertiginous advance for identifying the genomic sequence of SARS-CoV-2 allowed the development of a vaccine including mRNA-based vaccines, inactivated viruses, protein subunits, and adenoviral vaccines such as Sputnik. This study aims to report on autoimmune disease manifestations that occurred following COVID-19 Sputnik vaccination. Patients and Methods: A retrospective study was conducted on patients with new-onset autoimmune diseases induced by a post-COVID-19 vaccine between March 2021 and December 2022, in two referral hospitals in Mexico City and Argentina. The study evaluated patients who received the Sputnik vaccine and developed recent-onset autoimmune diseases. Results: Twenty-eight patients developed recent-onset autoimmune diseases after Sputnik vaccine. The median age was 56.9 ± 21.7 years, with 14 females and 14 males. The autoimmune diseases observed were neurological in 13 patients (46%), hematological autoimmune manifestations occurred in 12 patients (42%), with thrombotic disease observed in 10 patients (28%), and autoimmune hemolytic anemia in two patients (7.1%). Rheumatological disorders were present in two patients (7.1%), and endocrine disorders in one patient (3.5%). Principio del formulario Conclusion: Although the COVID-19 Sputnik vaccine is generally safe, it can lead to adverse effects. Thrombosis and Guillain-Barre were the most frequent manifestations observed in our group of patients.
Keywords: Autoimmune/Inflammatory Syndrome Induced by Adjuvants (ASIA); COVID-19 vaccines; adverse events; sputnik vaccines; systemic autoimmune diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of a rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomized controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. - DOI - PMC - PubMed
-
- Erra L., Uriarte I., Colado A., Paolini M.V., Seminario G., Fernández J.B., Tau L., Bernatowiez J., Moreira I., Vishnopolska S., et al. COVID-19 Vaccination Responses with Different Vaccine Platforms in Patients with Inborn Errors of Immunity. J. Clin. Immunol. 2022;43:271–285. doi: 10.1007/s10875-022-01382-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

