GDF8 Contributes to Liver Fibrogenesis and Concomitant Skeletal Muscle Wasting
- PMID: 37509548
- PMCID: PMC10377408
- DOI: 10.3390/biomedicines11071909
GDF8 Contributes to Liver Fibrogenesis and Concomitant Skeletal Muscle Wasting
Abstract
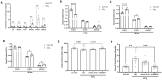
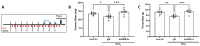
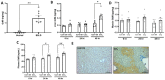
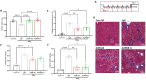
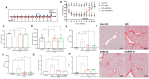
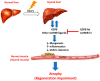
Patients with end-stage liver disease exhibit progressive skeletal muscle atrophy, highlighting a negative crosstalk between the injured liver and muscle. Our study was to determine whether TGFβ ligands function as the mediators. Acute or chronic liver injury was induced by a single or repeated administration of carbon tetrachloride. Skeletal muscle injury and repair was induced by intramuscular injection of cardiotoxin. Activin type IIB receptor (ActRIIB) ligands and growth differentiation factor 8 (Gdf8) were neutralized with ActRIIB-Fc fusion protein and a Gdf8-specific antibody, respectively. We found that acute hepatic injury induced rapid and adverse responses in muscle, which was blunted by neutralizing ActRIIB ligands. Chronic liver injury caused muscle atrophy and repair defects, which were prevented or reversed by inactivating ActRIIB ligands. Furthermore, we found that pericentral hepatocytes produce excessive Gdf8 in injured mouse liver and cirrhotic human liver. Specific inactivation of Gdf8 prevented liver injury-induced muscle atrophy, similar to neutralization of ActRIIB ligands. Inhibition of Gdf8 also reversed muscle atrophy in a treatment paradigm following chronic liver injury. Direct injection of exogenous Gdf8 protein into muscle along with acute focal muscle injury recapitulated similar dysregulated muscle regeneration as that observed with liver injury. The results indicate that injured liver negatively communicate with the muscle largely via Gdf8. Unexpectedly, inactivation of Gdf8 simultaneously ameliorated liver fibrosis in mice following chronic liver injury. In vitro, Gdf8 induced human hepatic stellate (LX-2) cells to form a septa-like structure and stimulated expression of profibrotic factors. Our findings identified Gdf8 as a novel hepatomyokine contributing to injured liver-muscle negative crosstalk along with liver injury progression.
Keywords: Gdf8; TGFβ family; liver injury; liver–muscle crosstalk; muscle atrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lee Y.H., Kim S.U., Song K., Park J.Y., Kim D.Y., Ahn S.H., Lee B.W., Kang E.S., Cha B.S., Han K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

